It is estimated that 26 million people worldwide and 6.2 million U.S. adults suffer from heart failure. Owing to the fact that not all of the patients can benefit from a heart transplant many patients have to wait for an available organ., and, in the case of severe heart failure, the wait is detrimental to one’s health. As a result, many of the people on the transplant lists die due to long waiting periods. This is because the demand for heart transplants is higher than the l supply. The average number of heart transplants done annually across the globe is less than 6,000, yet the U.S alone has approximately 100,000 potential patients.
The efficacy of heart transplants in managing end-stage heart failure is well established. Unfortunately, the limited availability of donor hearts translates into protracted wait times for numerous patients several of whom do not survive the waiting period. For example, the American Heart Association reports that 18.7 percent of the total number of patients that need donation die during such waiting. For this reason, it is critical to explore alternative solutions that can be used by an individual in the time before a donor’s organ delivery or as a permanent solution. One of these options is the so-called Total Artificial Heart.
|
State |
Waiting List (Total) |
|
Texas |
381 |
|
New York |
327 |
|
California |
264 |
|
Florida |
216 |
|
Illinois |
173 |
(Source: SRTR Annual Data Report, 2022)
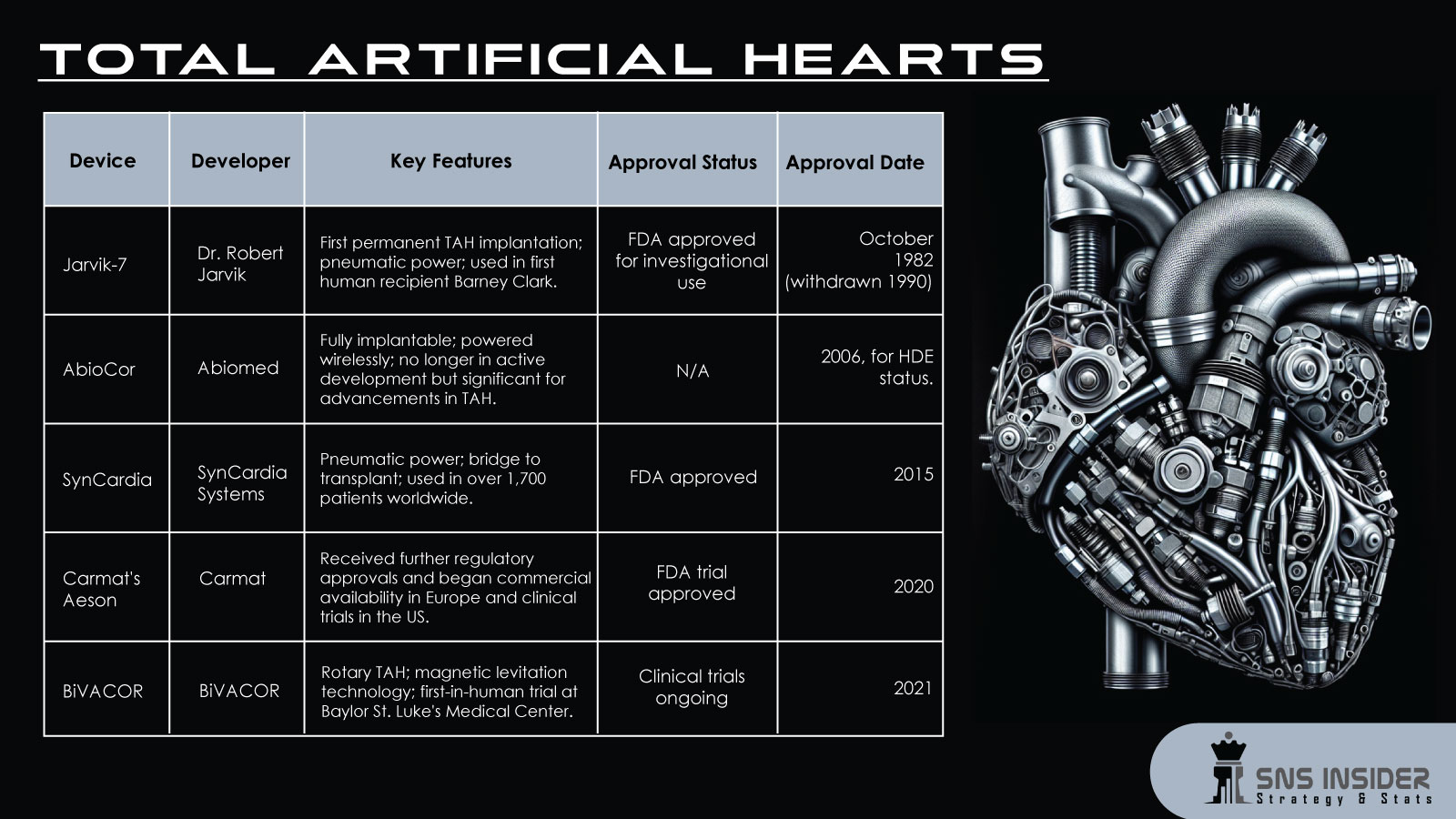
|
Device |
Developer |
Key Features |
Approval Status |
Approval Date |
|
Jarvik-7 |
Dr. Robert Jarvik |
First permanent TAH implantation; pneumatic power; used in first human recipient Barney Clark. |
FDA approved for investigational use |
October 1982 (withdrawn 1990) |
|
AbioCor |
Abiomed |
Fully implantable; powered wirelessly; no longer in active development but significant for advancements in TAH. |
N/A |
2006, for HDE status. |
|
SynCardia |
SynCardia Systems |
Pneumatic power; bridge to transplant; used in over 1,700 patients worldwide. |
FDA approved |
2015 |
|
Carmat's Aeson |
Carmat |
Received further regulatory approvals and began commercial availability in Europe and clinical trials in the US. |
FDA trial approved |
2020 |
|
BiVACOR |
BiVACOR |
Rotary TAH; magnetic levitation technology; first-in-human trial at Baylor St. Luke's Medical Centre. |
Clinical trials ongoing |
2021 |
CARMAT's Aeson TAH, which received CE marking in Europe and FDA approval in the United States in 2020. The product imitates the natural function of the human heart and is designed using biocompatible materials. The device’s adaptive heart rate changes depending on the activity that the patient is engaged in, facilitating a more accurate imitation of the user’s heart. Long-term artificial total heart replacement is intended for patients with end-stage heart failure.
An artificial heart of the new generation has introduced a heart replacement device designed using magnetic levitation, similar to that used in high-speed trains. Recently, the TAH was set in motion within a first-in-human trial at Baylor St. Luke’s Medical Centre. The impeller that delivers blood through the pulmonary and systemic circulatory systems on both sides is set in motion by a dual-sided centrifugal impeller. This design significantly reduces mechanical wear and blood trauma and improves the emergence of a resistant solution.
Several other companies are developing innovative TAH solutions:
The introduction of advanced TAHs like BiVACOR’s and CARMAT’s is poised to revolutionize patient care in several ways:
BiVACOR and CARMAT’s recent breakthroughs justifiably receive much attention. As their clinical trials are still in progress and the devices still have much potential for adoption, and they are likely to claim a large share of the market for cardiovascular devices, an increase in IHCA applications is expected to have a strong positive effect on their financial performance and forecasts. In conclusion, total Artificial Hearts are one of the prospective solutions to the severe problems in heart transplantation. They are, in particular, both satisfied to be the gap in the availability of donor organs. These devices are critical tools to incorporate advanced technologies as well as innovative designs for designing mechanisms for appropriate support of patients with terminal heart failure; such companies as BiVACOR and CARMAT are working on them. With the upcoming advances, such devices will have a dramatic impact on a decade of cardiovascular medicine. Would it be a joint problem to add provision of these services to a new era in cardiac care, and what could be possible to address any possible challenges for implementation, such are some of the questions that remain unclear to me at all.
Contact Us:
Akash Anand – Head of Business Development & Strategy
info@snsinsider.com
Phone: +1-415-230-0044 (US)