Clinical Chemistry Analyzer Market Report Scope & Overview:
To Get More Information on Clinical Chemistry Analyzer Market - Request Sample Report
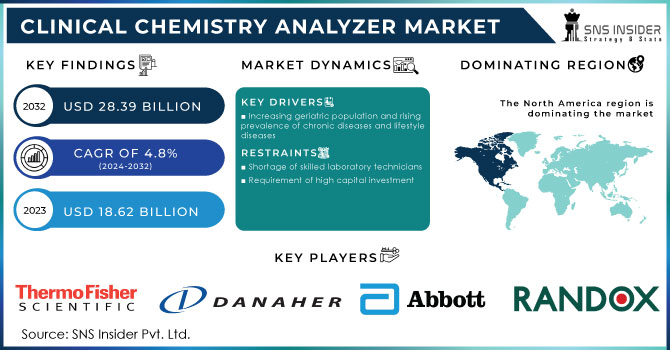
The Clinical Chemistry Analyzer Market size was estimated at USD 15.82 billion in 2023 and is expected to reach USD 24.27 billion by 2032 with a growing CAGR of 4.89% during the forecast period of 2024-2032. This study emphasizes sales and installed base of clinical chemistry analyzers by geography, presenting trends in adoption levels driven by healthcare infrastructure and diagnostic testing demand. The research reviews healthcare expenditure on clinical chemistry and explores regional disparities and the contribution of government expenditure in driving lab capacity growth. It also reviews technological innovations and adoption patterns, focusing on automation, incorporation of AI-based diagnostics, and movement toward high-throughput systems. Pricing analysis and cost trends are also evaluated in light of factors like manufacturing breakthroughs, affordability issues, and the influence of competitive pricing plans on market growth.
Market Dynamics
Drivers
-
The increasing prevalence of chronic diseases such as diabetes, cardiovascular disorders, and kidney diseases
The International Diabetes Federation (IDF) estimates that in 2021 approximately 537 million adults aged between 20-79 years suffered from diabetes, and this is anticipated to grow to 643 million by 2030. All these conditions must be continuously monitored using blood chemistry tests such as glucose, lipid profile, and kidney function tests, stimulating demand for analyzers. Besides, technological developments in automation and implementation of AI within clinical chemistry analyzers have enhanced test efficiency through decreased turnaround time and enhanced uptake by hospitals and diagnostic laboratories. The momentum toward preventive healthcare and regular health check-ups also augments market expansion. Furthermore, growth in laboratory automation is streamlining workflow, with organizations such as Roche and Abbott developing continuously to enhance testing accuracy and speed.
Restraints
-
The high cost of clinical chemistry analyzers and associated maintenance expenses pose significant market restraints.
Automated analyzers that operate in an automatic mode will be in the range of USD 30,000 to USD 200,000 and, as such, an expensive input for smaller-sized hospitals and clinics. Repeating expenditure on reagents, calibrations, and software revision further strains the facilities. Healthcare expenditures are reduced by budget restraints in LMIC countries as government-provided healthcare money cannot cover vast diagnostics infrastructure at this stage. World Health Organization (WHO) estimates that 40% of the world's population is deprived of access to basic diagnostics, citing financial constraints. Additionally, low reimbursement policies in some areas lead to high out-of-pocket costs, deterring patients from frequent diagnostic testing, and ultimately impacting market growth.
Opportunities
-
The shift toward point-of-care (POC) testing and personalized medicine presents significant growth opportunities for the Clinical Chemistry Analyzer Market.
The growing popularity of point-of-care (POC) testing and developments in personalized medicine are fueling emerging opportunities in the Clinical Chemistry Analyzer Market. Portable, easy-to-use analyzers are increasingly demanded in emergency rooms, ambulatory care, rural clinics, and home healthcare for quick diagnostics independent of centralized laboratories. This transition is enabled by developments in microfluidics and biosensors that allow small-scale analyzers to provide high-precision, real-time test results. Also, the development of personalized medicine, where treatment strategies are customized according to a person's biomarker profile, is fueling the need for specialized chemistry tests. Pharmaceutical companies and biotech companies are heavily investing in biomarker-based diagnostics, opening doors to new clinical chemistry uses. For instance, partnerships between Abbott and research organizations have catapulted the advancement of targeted diagnostics, making disease management more efficient.
Challenges
-
The Clinical Chemistry Analyzer Market faces significant challenges in terms of regulatory approvals and standardization across different regions.
Stringent regulations imposed by organizations such as the U.S. FDA, European Medicines Agency (EMA), and China's National Medical Products Administration (NMPA) establish lengthy approval procedures, slowing down product launches. For instance, in 2021, Roche encountered regulatory setbacks in the launch of its next-generation chemistry analyzer due to conformity issues associated with reagent validation. Furthermore, the difference in standards across regions presents hurdles in harmonizing quality control processes and test outcomes. Laboratories also face difficulties in cross-platform compatibility since reagent formulations and calibration procedures vary across manufacturers. Data integration in healthcare IT systems is another vital challenge, given that most analyzers continue to use legacy infrastructure, which causes inefficiencies in electronic health record (EHR) integration. The absence of trained professionals to run sophisticated analyzers, especially in developing areas, makes market growth even more challenging. Overcoming these technical and regulatory hurdles involves a lot of investment in compliance programs and training initiatives.
Key Segmentation
By Product
In 2023, the reagents segment dominated the clinical chemistry analyzer market with the highest revenue share of 57.8% due to the ever-increasing demand for reagents in regular diagnostic testing and their requirement for continuous replenishment. Reagents form a vital component of clinical chemistry tests, and their repetitive usage renders them an essential revenue-generating segment. The growing incidence of chronic diseases like diabetes, cardiovascular ailments, and renal disease has tremendously improved the demand for tests, thereby accelerating the growth of the reagents segment. Furthermore, the usage of automated and high-throughput analyzers has contributed to rising reagent utilization, adding to its market dominance. Conversely, the analyzers segment is expected to grow at the highest rate during the forecast period due to advancements in technology, laboratory automation, and the increasing demand for effective diagnostic solutions. Artificial intelligence and machine learning integration into clinical chemistry analyzers are enhancing diagnostic accuracy and efficiency, leading to these products becoming more desirable for healthcare institutions. In addition, the increasing emphasis on point-of-care testing and decentralized diagnostics is driving the use of small and portable analyzers, further propelling the segment's swift growth in the coming years.
By Test
The basic metabolic panel (BMP) segment was the largest, with a market share of 29.1% in 2023, owing to its widespread application for evaluating overall metabolic health. BMP tests are traditionally conducted to monitor kidney function, blood glucose, and electrolytes, thus they are a prerequisite in regular medical check-ups as well as for disease management. With the growing incidence of metabolic disorders like diabetes and kidney ailments, BMP tests have seen a huge surge in demand. These tests are also commonly ordered pre-operatively, during emergency cases, and to keep chronic patients under observation, which further increases their stronghold. The cost-effectiveness and fast turnaround time of BMP tests further make them the physician's and healthcare provider's first choice. Conversely, the specialty chemical tests segment is projected to be the fastest-growing segment, spurred by growing demand for targeted medicine and advanced biomarker testing. As the healthcare sector transitions to precision diagnostics, specialty chemical tests are gaining popularity in the detection of orphan diseases, monitoring certain biomarkers, and support of targeted therapies. The increasing emphasis on preventive healthcare and early disease diagnosis also helps drive the growth of this segment in the clinical chemistry analyzers market.
By End-use
In the end-use category, hospitals were the dominant segment in 2023 with a 58.4% market share due to their position as the main healthcare providers for inpatients and outpatients. They have sophisticated diagnostic laboratories, allowing them to perform a large number of tests for disease diagnosis, treatment monitoring, and preoperative evaluation. The rising number of admissions in hospitals for chronic disease, emergency care requirements, and surgery has fueled demand for clinical chemistry analyzers in hospitals. Also, hospitals provide the largest range of diagnostic services like biochemical analysis, hence becoming a prominent end-user of the market. However, the segment of diagnostic laboratories will expand at the highest growth rate during the forecast period, driven by the increasing demand for specialized diagnostic services and lab testing outsourcing. Most healthcare providers and solo practitioners are resorting to diagnostic laboratories because they are cost-saving, have rapid turnaround times, and specialize in complex testing procedures. The growth in freestanding diagnostic centers, coupled with greater awareness about preventive care among consumers, is driving further the expansion in this segment to be among the most attractive and promising areas for clinical chemistry analyzers.
Regional Analysis
North America dominated the market in 2023, driven by sophisticated healthcare infrastructure, large volumes of diagnostic testing, and the strong presence of major market players. The rising incidence of chronic diseases like diabetes and cardiovascular diseases, coupled with rising investments in laboratory automation, also fuels market growth in the region. Favorable reimbursement policies and high healthcare expenditures also play a role in its dominance.
Europe was close behind, driven by robust market growth due to well-developed healthcare systems, growing elderly populations, and rising demand for early disease detection. The UK, Germany, and France are leading because there is continuous development in laboratory technologies as well as encouraging support from regulatory bodies.
Asia-Pacific is set to witness the most rapid expansion, fueled by fast-paced urbanization, growing healthcare spending, and the mounting incidence of lifestyle diseases. China, India, and Japan are seeing rising demand for clinical chemistry analyzers as a result of increased diagnostic centers and government efforts to improve access to healthcare. The movement toward automation and electronic healthcare solutions further spurs regional market growth.
Do You Need any Customization Research on Clinical Chemistry Analyzer Market - Enquire Now
Key Players and Their Clinical Chemistry Analyzer Products:
-
Shenzhen Mindray Bio-Medical Electronics Co., Ltd. – BS-Series, CL-Series
-
Thermo Fisher Scientific, Inc. – Indiko Series, Konelab Series
-
Danaher Corporation (Beckman Coulter) – AU-Series, DxC Series
-
Abbott – ARCHITECT Series, Alinity Series
-
Siemens Healthineers AG – Atellica Series, ADVIA Chemistry Systems
-
F. Hoffmann-La Roche Ltd. – Cobas Series
-
Horiba, Ltd. – Pentra C-Series
-
ELITech Group – Selectra Series
-
QuidelOrtho Corporation – Vitros Series
-
Randox Laboratories Ltd. – RX Series
Recent Developments
In July 2024, Beckman Coulter Diagnostics launched the DxC 500i Clinical Analyzer, an integrated clinical chemistry and immunoassay analyzer designed to enhance efficiency in networked laboratory operations. The new system utilizes common reagents across Beckman Coulter’s portfolio, ensuring commutable patient results and optimized inventory management for hospitals and healthcare networks.
In June 2024, Siemens Healthineers introduced the Atellica CI Analyzer in the USA, a compact system designed for immunoassay and clinical chemistry testing. This advanced analyzer aims to enhance laboratory efficiency and address operational challenges in diagnostic testing.
| Report Attributes | Details |
| Market Size in 2023 | USD 15.82 Billion |
| Market Size by 2032 | USD 24.27 Billion |
| CAGR | CAGR of 4.89% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product [Analyzers (Small, Medium, Large, Very Large), Reagents (Calibrators, Controls, Standards, Others), Others] • By Test [Basic Metabolic Panel (BMP), Electrolyte Panel, Liver Panel, Lipid Profile, Renal Profile, Thyroid Function Panel, Specialty Chemical Tests] • By End-use [Hospitals, Academic Research Centers, Diagnostic Laboratories, Others] |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Thermo Fisher Scientific, Inc., Danaher Corporation (Beckman Coulter), Abbott, Siemens Healthineers AG, F. Hoffmann-La Roche Ltd., Horiba, Ltd., ELITech Group, QuidelOrtho Corporation, Randox Laboratories Ltd. |

