Hyperphosphatemia Treatment Market Report Scope & Overview:
To Get More Information on Hyperphosphatemia Treatment Market - Request Sample Report
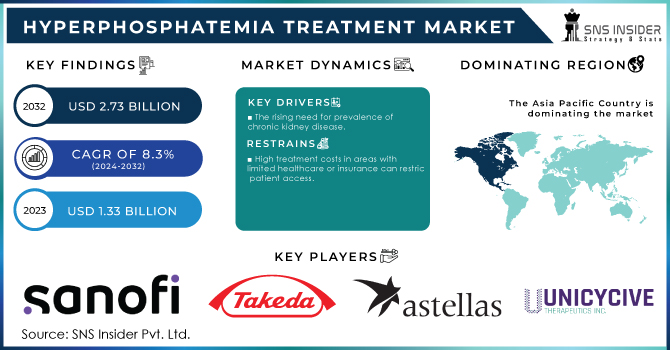
The Hyperphosphatemia Treatment Market Size was valued at USD 1.33 billion in 2023, and is expected to reach USD 2.73 billion by 2032, and grow at a CAGR of 8.3% over the forecast period 2024-2032.
A high phosphate concentration in the blood is referred to as hyperphosphatemia. Numerous medical issues, including kidney disease, particular drugs, and metabolic abnormalities, might contribute to it. In order to avoid problems, particularly in those with chronic kidney disease (CKD), the therapy of hyperphosphatemia frequently focuses on treating the underlying cause as well as controlling the phosphate levels. The following are some typical methods for treating hyperphosphatemia, dietary adjustments reducing consumption of phosphate-rich foods such dairy, processed meals, and soft beverages. Keeping the amount of phosphate absorbed from the digestive system under control by eating a low-phosphate diet.
Phosphate binders are drugs that assist in lowering phosphate absorption from the gastrointestinal system. They are frequently recommended to CKD patients who have problems excreting too much phosphorus. Sevelamer, lanthanum carbonate, and calcium-based binders (calcium carbonate, calcium acetate) are examples of common phosphate binders. Adjustments to medications: Your healthcare professional may change or stop the drug if hyperphosphatemia is brought on by that medicine. Treatment of Substantial Conditions: Phosphorus levels can be controlled by addressing the underlying causes of hyperphosphatemia, such as treating kidney disease.
Treatment for hyperphosphatemia, or increased phosphate levels in the blood, is largely necessary to ward off problems and maintain general health. An excessive amount of phosphate in the blood can cause calcium-phosphate crystals to form in soft tissues, such as blood vessels and joints. Joint discomfort and vascular calcification are two conditions that may be exacerbated by this. You can safeguard bone health and lower the risk of these consequences by treating hyperphosphatemia. Elevated phosphate levels can upset the body's calcium and phosphate balance, which is necessary for calcium regulation. The condition of the muscles, teeth, and bones may be impacted by this imbalance. The appropriate calcium-phosphate balance, which is essential for numerous physiological functions, can be maintained with effective treatment.
Market Dynamics
Driver
-
The rising need for prevalence of chronic kidney disease.
The market for treatments for hyperphosphatemia is largely driven by CKD. Hyperphosphatemia develops as CKD worsens because the kidneys become less effective in controlling phosphate levels. Treatments that control phosphate levels and related issues are in higher demand due to the rising prevalence of CKD around the world.
Restrain
In areas with insufficient healthcare resources or inadequate insurance coverage, high treatment prices may restrict access for some patient populations.
Opportunity
-
The rising clinical trials and the R&D related to the same.
The goal of ongoing research is to create new, more potent treatments for hyperphosphatemia. The development of cutting-edge treatments can promote market expansion and give patients better options.
Challenges
Although public and certain healthcare professionals are becoming more aware of kidney health and hyperphosphatemia, there is still a lack of broad understanding. Delays in diagnosis and treatment beginning may result from this.
Impact of Recession
Healthcare systems may have budgetary restrictions and staffing reductions during recessions, resulting in decreased access to medical facilities and healthcare experts. Patients with hyperphosphatemia could experience delayed diagnosis, start of treatment, and follow-up care as a result of this. Individuals and families frequently have financial difficulties during economic downturns. The cost of drugs, phosphate binders, and other therapies needed to treat hyperphosphatemia may be prohibitive for patients. Reduced financial resources may cause patients to abandon their treatment plans. Patients who were already receiving treatment for hyperphosphatemia may suffer changes if their healthcare facility or provider encounters financial difficulties during a recession. The outcomes of treatment and the continuity of care may be impacted by this.
Impact of Russia-Ukraine War
The influence of the Russia-Ukraine conflict on the management of hyperphosphatemia may be complex and multifaceted, influencing different facets of patient care and healthcare delivery. The effects would vary depending on the particular conditions, such as the intensity of the war, the location, the state of the healthcare system, and the available resources. Here are a few possible effects to think about. Health care facilities and infrastructure may be damaged or interfered with in areas that are directly impacted by the conflict. This could make it difficult to provide necessary medical services, such as therapy for hyperphosphatemia. Due to disturbances in supply chains and transportation due to conflict, there may be a shortage of drugs, including phosphate binders and other therapies for hyperphosphatemia.
Key Market Segmentation
By Product
-
Sevelamer
-
Calcium-Based phosphate Binders
-
Iron-Based phosphate Binders
-
Lanthanum Carbonate
-
Others
-
Non-Phosphate Binders
By Distribution Channel
-
Hospital Pharmacy
-
Retail Pharmacy
-
Online Stores
Regional Coverage
North America
-
US
-
Canada
-
Mexico
Europe
-
Eastern Europe
-
Poland
-
Romania
-
Hungary
-
Turkey
-
Rest of Eastern Europe
-
-
Western Europe
-
Germany
-
France
-
UK
-
Italy
-
Spain
-
Netherlands
-
Switzerland
-
Austria
-
Rest of Western Europe
-
Asia Pacific
-
China
-
India
-
Japan
-
South Korea
-
Vietnam
-
Singapore
-
Australia
-
Rest of Asia Pacific
Middle East & Africa
-
Middle East
-
UAE
-
Egypt
-
Saudi Arabia
-
Qatar
-
Rest of Middle East
-
-
Africa
-
Nigeria
-
South Africa
-
Rest of Africa
-
Latin America
-
Brazil
-
Argentina
-
Colombia
-
Rest of Latin America
Regional Analysis
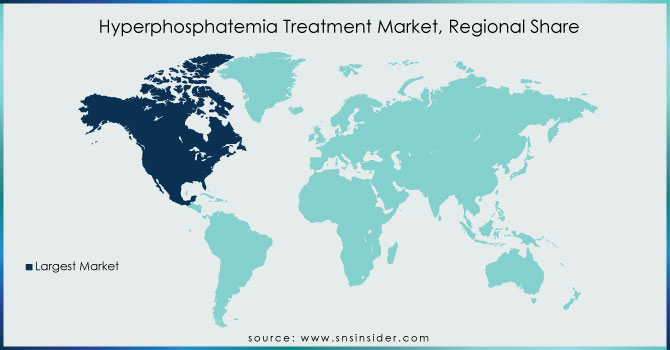
North America will be dominating the market because in general, North America has access to highly developed healthcare systems and cutting-edge medical procedures. Phosphate binders are among the treatments available to patients with hyperphosphatemia, along with regular monitoring. Options for advanced instances include dialysis and kidney transplants. Dietary advice is frequently included in treatment strategies.
Europe will be the region with the second largest share because the accessibility of treatment is impacted by the combination of public and private healthcare systems offered by European nations. Patients can obtain cutting-edge therapies, albeit there may be differences between Eastern and Western Europe. Perhaps more countries with advanced technology are offering telehealth services. Changing one's diet and way of life are frequently highlighted.
APAC region will be the region with the highest CAGR growth rate because access to cutting-edge therapies may be restricted in some areas of Asia, but major urban areas frequently offer state-of-the-art medical facilities. Some countries' treatment philosophies may be influenced by traditional medical practices. The availability of treatments and their options vary between nations.
Do You Need any Customization Research on Hyperphosphatemia Treatment Market - Enquire Now
Key Players
The major players are Sanofi, Takeda Pharmaceutical Company, Astellas Pharma, Ardelyx, Unicycive Therapeutics, Keryx Biopharmaceuticals, Zeria Pharmaceuticals, Vifor Pharma Management, Lupin Limited others.
| Report Attributes | Details |
| Market Size in 2023 | US$ 1.33 Bn |
| Market Size by 2032 | US$ 2.73 Bn |
| CAGR | CAGR of 8.3% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product (Sevelamer, Calcium-Based phosphate Binders, Iron-Based phosphate Binders, Lanthanum Carbonate, Others, Non-Phosphate Binders) • By Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Stores) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]). Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia Rest of Latin America) |
| Company Profiles | Sanofi, Takeda Pharmaceutical Company, Astellas Pharma, Ardelyx, Unicycive Therapeutics, Keryx Biopharmaceuticals, Zeria Pharmaceuticals, Vifor Pharma Management, Lupin Limited |
| Key Drivers | • The rising need for prevalence of chronic kidney disease. |
| Market Restraints | • In areas with insufficient healthcare resources or inadequate insurance coverage, high treatment prices may restrict access for some patient populations. |

