Safety Lancets Market Report Scope & Overview:
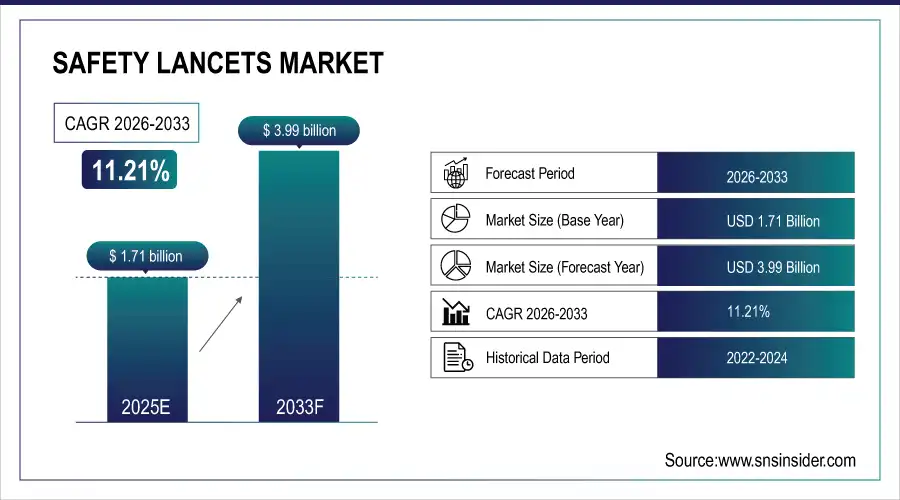
The Safety Lancets Market size is valued at USD 1.71 Billion in 2025E and is expected to reach USD 3.99 Billion by 2033 and grow at a CAGR of 11.21% over the forecast period 2026-2033.
The Safety Lancets Market analysis, driven by the rising global prevalence of chronic diseases, especially diabetes, which necessitates frequent blood glucose monitoring and thereby fuels consistent demand for safety lancets in both clinical and home settings.
According to study, 43% of people with diabetes (252 million adults) are undiagnosed worldwide, meaning a large population still requires screening and testing services.
Market Size and Forecast:
-
Market Size in 2025: USD 1.71 Billion
-
Market Size by 2033: USD 3.99 Billion
-
CAGR: 11.21% from 2026 to 2033
-
Base Year: 2025
-
Forecast Period: 2026–2033
-
Historical Data: 2022–2024
To Get more information On Safety Lancets Market - Request Free Sample Report
Safety Lancets Market Trends:
-
Rising global diabetes prevalence boosts demand for safe blood sampling devices.
-
Increasing adoption of home healthcare drives usage of user-friendly safety lancets.
-
Telemedicine expansion encourages frequent at-home blood glucose monitoring and self-testing.
-
Growing awareness of preventive healthcare promotes regular monitoring and lancet utilization.
-
Integration with digital devices and apps enables smarter, connected monitoring solutions
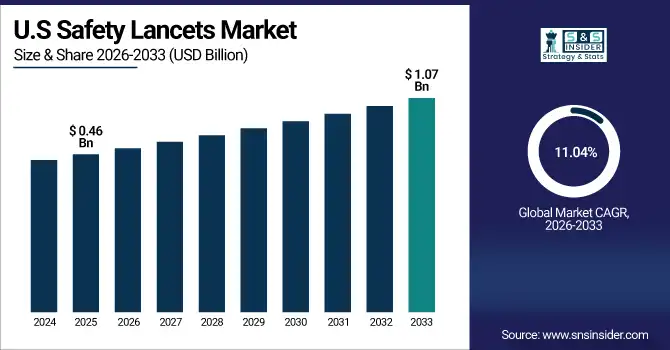
The U.S. Safety Lancets Market size is USD 0.46 Billion in 2025E and is expected to reach USD 1.07 Billion by 2033, growing at a CAGR of 11.04% over the forecast period of 2026-2033,
The U.S. market is driven by high diabetes prevalence, advanced healthcare infrastructure, and stringent safety regulations. Widespread adoption of home monitoring and point-of-care diagnostics, along with rising patient awareness and technological innovations in ergonomic and digital-integrated lancets, ensures strong demand in hospitals, clinics, and home-care settings.
Safety Lancets Market Growth Drivers:
-
Rising Global Prevalence of Diabetes and Chronic Diseases Fuels Safety Lancet Demand
The primary driver for the Safety Lancets Market is the rapidly increasing global prevalence of diabetes and other chronic diseases that require frequent blood monitoring. According to the International Diabetes Federation, nearly 589 million adults worldwide are living with diabetes, and this number is expected to rise sharply in the coming decades. Regular blood glucose monitoring is critical for managing the disease, and safety lancets are indispensable in both clinical and home settings. Their safety features minimize needlestick injuries and cross-contamination, which is especially important for healthcare workers and patients performing self-testing.
People with diabetes typically test their blood glucose 2–4 times daily, which translates to an estimated 730–1,460 lancets per patient annually, underlining the recurring demand.
Safety Lancets Market Restraints:
-
High Cost and Single-Use Design Limits Adoption in Price-Sensitive Markets
One major restraint limiting the market growth is the relatively higher cost of safety lancets compared to conventional lancets and their single-use design. While these devices are safer, the cost can be a barrier, particularly in low- and middle-income regions where large portions of the population cannot afford frequent self-monitoring. Hospitals and clinics must also manage procurement budgets, and the continuous replacement requirement for single-use lancets adds to operational expenses. Additionally, some safety lancets require specialized disposal methods to comply with biohazard regulations, further increasing costs.
Safety Lancets Market Opportunities:
-
Growing Adoption of Home Healthcare and Point-of-Care Diagnostics
A major opportunity for the market lies in the expanding home healthcare and point-of-care diagnostics sector. Patients are increasingly seeking convenient, at-home testing solutions for chronic disease management, driven by lifestyle changes, busy schedules, and the need for continuous monitoring. Safety lancets with ergonomic designs, minimal pain, and integration with digital glucometers present a strong growth opportunity. Additionally, emerging markets in Asia-Pacific, Latin America, and the Middle East are witnessing rising healthcare infrastructure and awareness, creating new avenues for safety lancet adoption.
Safety lancets integrated with digital glucometers and mobile apps are gaining adoption, with 35–40% of newly sold lancets in developed markets now being compatible with smart monitoring devices.
Safety Lancets Market Segmentation Analysis:
-
By Type: In 2025, Button-activated devices led the market with a share of 56.48%, while Pressure/Contact-activated is the fastest-growing segment with a CAGR of 12.14%.
-
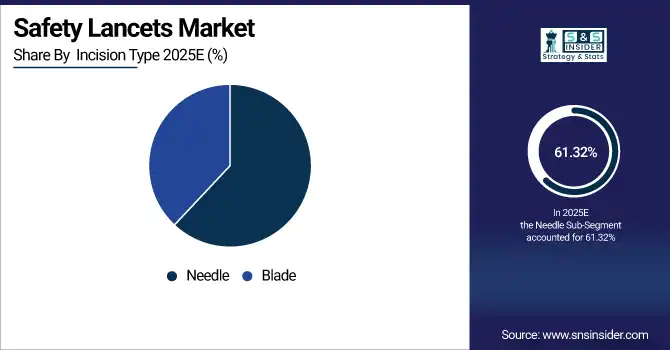
By Incision Type: In 2025, Needle-based devices led the market with a share of 61.32%, while Blade-based devices are the fastest-growing segment with a CAGR of 11.85%.
-
By Application: In 2025, Blood Glucose Monitoring led the market with a share of 42.15%, while Hemoglobin Testing is the fastest-growing segment with a CAGR of 12.05%.
-
By End-user: In 2025, Hospitals & Clinics led the market with a share of 58.94%, while Home Care is the fastest-growing segment with a CAGR of 12.72%.
By Type, Button-activated Lead Market and Pressure/Contact-activated Fastest Growth
Button-activated lancets dominate the market due to their ease of use, consistent penetration depth, and reduced pain, making them preferred in clinical and home settings. Their ergonomic design and reliability for repetitive testing ensure higher adoption compared to pressure/contact-activated lancets, driving steady demand across hospitals and self-monitoring users.
Pressure/contact-activated lancets are the fastest-growing segment, especially in emerging markets, due to their cost-effectiveness and portability. These lancets appeal to budget-conscious users and home-care patients seeking simple, reliable devices. Increased awareness of self-monitoring and preventive healthcare is accelerating their adoption in both developing and urban regions.
By Incision Type, Needle Lead Market and Blade Fastest Growth
Needle-based lancets remain dominant due to of their precision, rapid blood collection, and compatibility with most glucometers. Their proven safety and minimal training requirement make them a standard choice in hospitals, clinics, and home-use scenarios, ensuring consistent market demand and reinforcing their position as the most widely used incision type.
Blade-based lancets are witnessing the fastest growth due to innovations in pain-free designs and ergonomic handling, attracting home-care users seeking minimal discomfort. Increased patient awareness about painless testing, along with technological integration with digital glucometers, drives their adoption across emerging and developed markets.
By Application, Blood Glucose Monitoring Lead Market and Hemoglobin Testing Fastest Growth
Blood glucose monitoring dominates the market as diabetes prevalence rises globally. Safety lancets are crucial for frequent daily testing, making this application the primary driver of revenue. Hospitals, clinics, and home-care patients rely heavily on lancets for accurate, safe, and repeated glucose testing.
Hemoglobin testing is the fastest-growing segments due to rising anemia awareness and preventive screening programs, especially in emerging regions. Point-of-care testing for hemoglobin allows rapid results, driving adoption of safety lancets for schools, clinics, and home monitoring, creating a new revenue stream beyond traditional glucose monitoring.
By End-user, Hospitals & Clinics Lead Market and Home Care Fastest Growth.
Hospitals and clinics dominate end-user adoption due to their high patient throughput and regulatory compliance requirements. Institutional use ensures repeated and large-scale procurement of safety lancets for blood glucose and other tests, establishing hospitals and clinics as the primary revenue source in both developed and emerging markets.
Home care is the fastest-growing segment as patients increasingly prefer self-monitoring for convenience, privacy, and time-saving. Rising telemedicine adoption, awareness of chronic disease management, and easy-to-use lancet designs fuel growth in this segment, especially among diabetic patients managing their condition outside hospital environments.
Safety Lancets Market Regional Analysis:
North America Safety Lancets Market Insights:
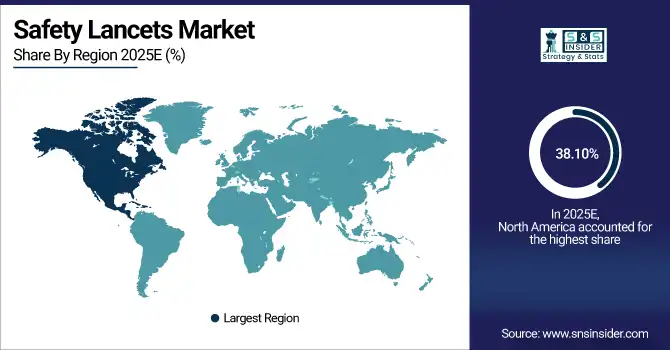
The North America dominated the Safety Lancets Market in 2025E, with over 38.10% revenue share, due to the high prevalence of diabetes, advanced healthcare infrastructure, and strict safety regulations. Hospitals and clinics heavily invest in safety-engineered lancets to minimize needlestick injuries and improve patient care. Widespread adoption of home healthcare and self-monitoring programs further fuels demand. Technological innovations, strong distribution networks, and awareness campaigns drive consistent market growth. The U.S. and Canada are key contributors, with government initiatives supporting chronic disease management and preventive healthcare.
Get Customized Report as per Your Business Requirement - Enquiry Now
U.S and Canada Safety Lancets Market Insights
The U.S. and Canada dominate the market due to high diabetes prevalence, advanced healthcare systems, and strict safety regulations. Widespread use of home monitoring, hospital demand, and technological innovations in ergonomic and smart lancets drive consistent growth, making North America a key revenue-generating region.
Asia Pacific Safety Lancets Market Insights:
The Asia Pacific region is expected to have the fastest-growing CAGR 12.14%, due to rising diabetes prevalence, expanding healthcare access, and increasing disposable incomes. Countries like China and India are witnessing rapid adoption of self-monitoring and home diagnostics solutions. Emerging healthcare infrastructure, government health programs, and growing awareness of preventive care contribute to the surge in demand. The preference for cost-effective and ergonomic safety lancets in both urban and rural areas further accelerates market growth in the region.
China and India Safety Lancets Market Insights
China and India are the fastest-growing markets, driven by rising diabetes cases, expanding healthcare access, and increasing awareness of preventive care. Affordable and easy-to-use safety lancets for home and clinical use, combined with government initiatives, fuel strong adoption across urban and rural populations.
Europe Safety Lancets Market Insights
Europe maintains steady growth in the Safety Lancets Market, driven by strict regulatory standards, high patient awareness, and a focus on hospital safety protocols. The demand is fueled by well-established healthcare systems, aging populations, and widespread adoption of diabetes management programs. Technological advancements, such as pain-reducing and smart lancets integrated with digital glucometers, support market expansion. Countries like Germany, France, and the U.K. are key contributors, with home healthcare adoption complementing institutional usage to strengthen the overall regional market.
Germany and U.K. Safety Lancets Market Insights
The U.K. and Germany maintain steady market growth due to robust healthcare infrastructure, aging populations, and stringent safety protocols. Hospitals, clinics, and home-care adoption of advanced lancets, including digital-integrated devices, support sustained demand for both institutional and patient-led blood monitoring.
Latin America (LATAM) and Middle East & Africa (MEA) Safety Lancets Market Insights
Latin America and the Middle East & Africa show moderate growth, supported by rising healthcare infrastructure, increasing prevalence of diabetes, and awareness programs. Countries like Brazil, Mexico, Saudi Arabia, and South Africa are expanding access to home diagnostics and point-of-care testing. However, cost sensitivity and limited healthcare access in rural areas pose challenges. Despite this, government initiatives and partnerships with global medical device manufacturers are driving adoption of safety lancets in hospitals, clinics, and emerging home-care settings.
Safety Lancets Market Competitive Landscape:
Roche Diagnostics, a Switzerland-based leader, dominates the safety lancets segment through its Accu-Chek product line, offering reliable, pain-minimized, and user-friendly devices for blood glucose monitoring. With strong global distribution and integration with digital glucometers, Roche meets both clinical and home-care demands. Continuous innovation, regulatory compliance, and strategic partnerships reinforce its market leadership, especially in North America and Europe, addressing rising diabetes prevalence worldwide.
-
In March 2025, Roche Diagnostics launched a new single-use safety lancet with improved pain reduction and automatic retraction features, strengthening its portfolio in diabetes management and reinforcing its commitment to patient safety and innovation.
Terumo Medical Corporation, headquartered in Japan, is a key player in safety lancets, known for ergonomic, precise, and safe lancet solutions. The company focuses on both hospital and home-care markets, leveraging advanced manufacturing technology and quality standards. With expansion in Asia-Pacific and emerging markets, Terumo addresses the growing demand for cost-effective, reliable lancets for diabetes and other chronic disease monitoring.
-
In April 2025, Terumo launched a new safety lancet line with optimized ergonomics and single-use technology, supporting safer blood sampling practices and its strategic expansion in clinical and home healthcare markets.
Nipro Corporation, a leading Japanese medical device manufacturer, offers a wide range of safety lancets for blood glucose and point-of-care diagnostics. The company emphasizes innovation, minimal pain designs, and patient safety, catering to hospitals, clinics, and home users. Nipro’s strong presence in Asia-Pacific, along with regulatory-approved products and strategic collaborations, drives its growth in the expanding safety lancet market globally.
-
In June 2025, Nipro introduced new safety lancets with improved ergonomics and minimal discomfort, enhancing self-testing solutions and reinforcing its strategic focus on diabetes care.
Safety Lancets Market Key Players:
Some of the Safety Lancets Market Companies are:
-
Roche Diagnostics
-
Abbott Laboratories
-
Becton, Dickinson and Company
-
Terumo Medical Corporation
-
Cardinal Health
-
Greiner Bio-One International GmbH
-
HTL-STREFA S.A.
-
Sarstedt AG & Co. KG
-
Nipro Corporation
-
Improve Medical Technology Co. Ltd
-
Simple Diagnostics
-
Ypsomed AG
-
Owen Mumford Ltd.
-
Arkray Inc.
-
Perrigo Company plc
-
Smith’s Medical
-
Medline Industries
-
Omron Healthcare
-
McKesson Corporation
-
Bayer AG
| Report Attributes | Details |
|---|---|
| Market Size in 2025E | USD 1.71 Billion |
| Market Size by 2033 | USD 3.99 Billion |
| CAGR | CAGR of 11.21% From 2026 to 2033 |
| Base Year | 2025E |
| Forecast Period | 2026-2033 |
| Historical Data | 2022-2024 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | •By Type (Button-activated, Pressure/Contact-activated) •By Incision Type (Needle, Blade) •By Application (Blood Glucose Monitoring, Hemoglobin Testing, Cholesterol Testing, Others) •By End-user (Hospitals & Clinics, Home Care) |
| Regional Analysis/Coverage | North America (US, Canada), Europe (Germany, UK, France, Italy, Spain, Russia, Poland, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Australia, ASEAN Countries, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Mexico, Colombia, Rest of Latin America). |
| Company Profiles | Roche Diagnostics, Abbott Laboratories, Becton, Dickinson and Company, Terumo Medical Corporation, Cardinal Health, Greiner Bio-One International GmbH, HTL-STREFA S.A., Sarstedt AG & Co. KG, Nipro Corporation, Improve Medical Technology Co. Ltd, Simple Diagnostics, Ypsomed AG, Owen Mumford Ltd., Arkray Inc., Perrigo Company plc, Smiths Medical, Medline Industries, Omron Healthcare, McKesson Corporation, and Bayer AG |

