Human Papillomavirus (HPV) Vaccine Market Report Scope & Overview:
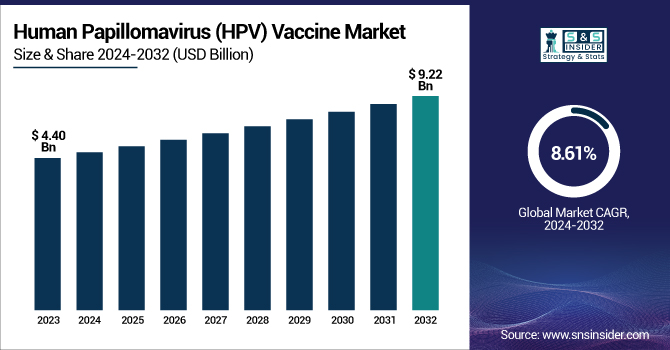
The human papillomavirus (HPV) vaccine market size was valued at USD 8.14 billion in 2024 and is expected to reach USD 27.36 billion by 2032, growing at a CAGR of 16.38% from 2025 to 2032.
To Get More Information on Human Papillomavirus (HPV) Vaccine Market - Request Sample Report
The global human papillomavirus (HPV) vaccine market is being driven by the increasing prevalence of HPV associated diseases across the world and the advent of vaccination programs to curb these cases. With one in three men around the world infected with genital HPV, the WHO noted the need for general vaccination. HPV causes almost 95% of cervical cancers and also has associations with oropharyngeal, anal, and penile cancers. Nearly 36,000 cases of HPV-related cancers occur each year in the U.S., the CDC says. Global vaccination coverage is inequitable.
In January 2025, the Indian government announced that the nationwide rollout of the HPV vaccine will begin in a few months, focusing on adolescent girls to curb cervical cancer rates.
For instance, 2022 WHO data showed that only 21% of girls received the full vaccine course worldwide, underscoring a missed opportunity. Higher government and private investments in preventive healthcare, supportive regulatory approvals, and R&D investments made by companies like Merck & Co. and GlaxoSmithKline, early entrants in the HPV vaccine market, have also contributed, in addition to market development. Gavi, the Vaccine Alliance, supported programs have also improved supply access in low- and middle-income countries. Increasing awareness and application in the national immunization schedule are other factors promoting the growth of the human papillomavirus (HPV) vaccine market. Strong clinical pipelines and the WHO’s call to eliminate cervical cancer will continue to boost the HPV vaccine market size in the future.
In January 2025, Ethiopia shifted to a single-dose HPV vaccination schedule, which significantly increased the number of vaccinated girls across the country.
HPV Vaccine Market Dynamics:
Drivers:
-
Rising Immunization Initiatives, Technological Advancements, and Government Support Propel Market Expansion
The growth of the human papillomavirus (HPV) vaccine market is driven by the increasing number of government-run national HPV vaccination programs, growing public awareness of HPV, and innovative product development. As of 2023, more than 125 countries have introduced the HPV vaccine into national immunization programs (WHO), resulting in substantial demand-side expansion.
Large pharmaceutical companies like BioNTech and Moderna are involved in developing the mRNA-based HPV vaccines, indicating increased R&D momentum. Available NIH funding data show that R&D for U.S.-based HPV vaccine projects has been awarded more than USD 50 million in federal grants over the past three years. Further boosting access is regulatory assistance like the FDA’s extended Gardasil 9 approval for people as old as 45. Supply-side conditions are also on the mend, as companies ramp up production to cope with expanding global demand, particularly in underserved areas. Global partnerships, including partnerships like those between UNICEF and vaccine manufacturers, help secure procurement and delivery in low-income countries, enhancing and leveraging the supply chain and access. These are drivers of long-range human papillomavirus (HPV) vaccine market share growth.
In January 2025, Bangladesh reported a 93% coverage rate in its national HPV vaccination program, highlighting the success of routine immunization efforts.
Restraints:
-
Supply Chain Barriers, Vaccine Hesitancy, and Uneven Global Coverage Impede Market Growth
While high-income countries have included HPV vaccines in their routine schedules, only 41% of low-income countries have an adequate supply, the group said, citing Gavi. Vaccine distribution is also a challenge and may be protracted in time and inefficient, due to the lack of availability of raw material, and cold-chain logistics for safe transport and storage, particularly in remote locations. Moreover, public mistrust and misinformation about vaccine safety continue to dampen vaccination uptake.
A 2022 study in JAMA Pediatrics found that 20% of parents in the U.S. were reluctant to vaccinate adolescents based on safety worries, even though the contrary was well-established. However, time-intensive regulatory approval and non-uniform implementation of guidelines within countries continue to stifle market expansion. For instance, in some low-income countries, there are undefined guidelines for age-based vaccine delivery or booster doses, which leads to limited continuity of coverage. The newer vaccines are prohibitively expensive for countries not funded through Gavi or WHO programs. In addition to this, vaccines are costly to develop and approve, with USD 100 m-plus price tags on clinical trials, limiting many smaller biotech firms’ participation in the market. Together, these hurdles have all but muted the otherwise robust prospects of the human papillomavirus (HPV) vaccine market.
Human Papillomavirus (HPV) Vaccine Market Segmentation Analysis:
By Type
Polyvalent HPV vaccines, mainly Merck’s Gardasil and Gardasil 9, contributed to a share of over 85% of the overall human papillomavirus (HPV) vaccine market in 2024. Their comprehensive coverage of multiple HPV strains, which include types 6, 11, 16, and 18 that are responsible for most HPV-related cancers and genital warts, renders them the choice vaccine for global consumption.
Concerning it, the bivalent vaccine, only against HPV types 16 and 18, Cervarix, developed by GSK, has faced decreased acceptance as the narrower protection spectrum of actions somehow lowers demand. The strongest segment of growth is also the polyvalent, and has followed a growth path influenced by enhanced regulatory clearances and extended age range indications (Adults up to 45 years of age) and by increased uptake in national vaccination programs.
By Disease Indication
The HPV-related cancer segment accounted for a major share of around 70% in 2024, and the rising importance of cancer prevention in the global healthcare programs and policies is contributing to its dominant share. The segment remains a focus of considerable investment in health care resources and attention because HPV is responsible for almost all cases of cervical cancer; as well, a high proportion of anal, oropharyngeal, and penile cancer cases. The most rapidly increasing section continues to be HPV-associated cancers, with rising cancer incidents, increased screening, and government-promoted cancer eradication program-related vaccinations.
By Distribution Channel
Hospitals and retail pharmacies dominated the market, holding a market share of around 60% in 2024. Being widely available and well integrated into routine public vaccination programs, they represent the most important distribution channel. The segment of hospitals and retail pharmacies is also growing at the fastest CAGR, driven by the growing availability of vaccines in outpatient care, the development of healthcare infrastructure in developing countries, and growing consumer confidence in institutional vaccination services. And these channels are now pivotal for adolescent and adult HPV vaccination efforts.
Human Papillomavirus Vaccine Market Regional Insights:
In 2024, North America remained at the forefront in the human papillomavirus vaccine market owing to the presence of a robust healthcare system, high vaccination coverage, stringent regulations, and organized vaccination schedules. The U.S. human papillomavirus (HPV) vaccine market size was valued at USD 3.44 billion in 2024 and is expected to reach USD 10.96 billion by 2032, growing at a CAGR of 15.62% from 2025 to 2032. The U.S. was leading, with widespread public-private vaccination campaigns having supported over 77% of adolescent girls and 74% of boys in receiving at least one dose of the HPV vaccine, according to CDC data. Also, robust government support, such as the Vaccines for Children (VFC) program, guarantees steady need and access. Canada is gradually advancing its vaccine campaign with school-based programs nationwide and gender-neutral immunization policies. In Mexico, where coverage rates are still climbing, regional cooperation, with the support of the WHO, is also helping.
The HPV vaccine market in APAC is estimated to grow at the fastest rate due to high cancer awareness, growing government-aided programs for immunization, and a large population of adolescents. India, with its millions of school-age girls, is a critical factor in the future national rollout of its HPV vaccine in 2025. Serum Institute’s less expensive vaccine, which is quadrivalent, is expected to improve access in both urban and rural areas. China is also advancing with pilot immunization, scaling up of public education, and Australia continues to lead the share of young people who are vaccinated in school-based programs, reaching over 80% among adolescents. Japan and South Korea are increasing the use of post-safety reevaluation of HPV vaccines.
Europe is a mature market, and growth has been somewhat slower because of saturation in high-income countries and vaccine hesitancy in some populations. Demand is highest in the UK, which just entered the market with over 85% coverage rates in school-based programs and a gender-neutral policy with benefits offered to boys. France and Germany have stepped up HPV education to thwart hesitancy, and Italy and Spain have broadened regional immunization policies. Eastern European nations such as Poland and Turkey are catching up with the help of EU funds and awareness campaigns. While there has been good initial coverage, misinformation and vaccine safety concerns have slightly held the adoption rates down in some countries.
LAMEA is witnessing a steady and promising growth, as more healthcare education, WHO-Gavi-backed initiatives, and government cancer prevention commitments are adopted. Brazil is the region’s front-runner, with vaccination rates of nearly 70% among schoolchildren, achieved through large, school-based vaccination campaigns and public awareness campaigns. Argentina is rapidly increasing its HPV vaccination and has multi-year plans on the road. In South Africa, in the Middle East & Africa region, public-sector vaccination initiatives of HPV vaccines are performed, and the use of HPV vaccines in school outreach programs is included.
Key Players in the Human Papillomavirus Vaccine Market
Prominent human papillomavirus (HPV) vaccine players operating in the market include Merck & Co., Inc., GlaxoSmithKline plc (GSK), Serum Institute of India Pvt. Ltd., Sanofi Pasteur SA, Pfizer Inc., Inovio Pharmaceuticals Inc., Walvax Biotechnology Co., Ltd., Bharat Biotech International Ltd., Johnson & Johnson Services, Inc., Moderna, Inc., and Gilead Sciences, Inc.
Recent Developments in HPV Vaccine Market
-
In January 2025, Merck is facing a USD 8 billion lawsuit related to Gardasil, with a jury trial underway addressing allegations about vaccine-related side effects.
-
In January 2025, GSK discontinued its Phase 2 HPV vaccine candidate due to a lack of best-in-class potential, halting further development.
| Report Attributes | Details |
| Market Size in 2024 | USD 8.14 billion |
| Market Size by 2032 | USD 27.36 billion |
| CAGR | CAGR of 16.38% From 2025 to 2032 |
| Base Year | 2024 |
| Forecast Period | 2025-2032 |
| Historical Data | 2021-2023 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Type (Bivalent, Polyvalent) • By Disease Indication (HPV Related Cancer, Genital Warts) • By Distribution Channel (Hospital & Retail Pharmacies, Government Suppliers, and Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, Poland, Turkey, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Rest of Latin America) |
| Company Profiles | Merck & Co., Inc., GlaxoSmithKline plc (GSK), Serum Institute of India Pvt. Ltd., Sanofi Pasteur SA, Pfizer Inc., Inovio Pharmaceuticals Inc., Walvax Biotechnology Co., Ltd., Bharat Biotech International Ltd., Johnson & Johnson Services, Inc., Moderna, Inc., and Gilead Sciences, Inc. |

