The Pediatric Catheter Market size was valued at USD 8.82 billion in 2024 and is expected to reach USD 18.01 billion by 2032, growing at a CAGR of 9.35% over the forecast period of 2025-2032.

To Get more information on Pediatric Catheter Market - Request Free Sample Report
Increasing clinical need resulting from an emerging children's population and increasing prevalence of chronic conditions is fueling tremendous momentum in the pediatric catheter market growth. Approximately 8 million children are born each year with congenital defects, and many of them need assistance with catheterization (WHO). Approximately 1 out of 110 births in the U.S. leads to congenital heart defects (CHD), fueling demand for pediatric cardiovascular catheters and associated procedures (CDC). The high rate of growth in pediatric urinary tract infections, over 7% of febrile illnesses in children below two years, goes a long way in contributing to the increase in the size of the pediatric urinary catheter market.
In clinical trials, a study in 2024 revealed that the use of AI tools for catheter placement and tracking their performance can decrease catheter-related bloodstream infections (CRBSIs) in children by over 30%.
Delayed diagnosis due to healthcare disparities and weak newborn screening programs in developing countries propels demand for advanced neonatal and infant catheters. The Spina Bifida Association asserts that restricted insurance coverage leads 60% of American pediatric catheter users to incur significant out-of-pocket costs, impacting market access. Over 400,000 American children also suffer from neurogenic bladders, typically requiring life-long use of pediatric central venous catheters and pediatric medical catheters (Children's National, Yale Medicine).
With the pediatric device consortia of the FDA providing more than USD 6 million annually in grants to support pediatric-specific device development, regulatory assistance is limiting the global pediatric catheter market. Increasing R&D expenditure has led to innovations in pediatric catheterization products, such as antimicrobial coating and artificial intelligence-based monitoring systems to reduce procedure risk and rates of infection. The demand for minimally invasive pediatric surgeries has increased by over 15% over the last five years, contributing to the growth of the pediatric catheter market as well.
Robust action in catheter material innovations and device miniaturization is evident in pediatric catheter market trends. Additionally, although developing countries are poised to experience rapid adoption with expanding healthcare infrastructure spending, the U.S. pediatric catheter market is a hub for technical advancement and regulatory oversight.
Product diversification and infection control are the prime focuses of global and specialty pediatric catheter market companies that dominate the pediatric catheter market share. The present market research is predominantly centered around government-sponsored activities, clinical trials, and strategic partnerships.
A 2023 case study on VP shunt catheter migration encouraged key stakeholders to invest in anchoring system redesigns, thus creating safer and more reliable pediatric medical catheters for neurosurgical applications.
Drivers:
Increasing Surgical Volumes, Healthcare Infrastructure Development, and Public-Private Investment Collaborations Drive Market Growth
Increased pediatric surgeries associated with trauma, cancer, and intensive care procedures are among the primary driving forces for the pediatric catheter market. The Journal of Pediatric Surgery estimates that, in the U.S. alone, nearly 200,000 pediatric surgeries are performed annually, which require temporary or indwelling pediatric catheters. As interest in enhancing pediatric ICU care picks up, the application of pediatric central venous catheters is on the rise, especially among preterm infants and chemotherapy patients.
Moreover, the number of children's hospitals and NICUs in developing countries is rising as a result of increased catheter utilization. Strategic investments by organizations, such as the Bill & Melinda Gates Foundation, are driving funds into child health innovation platforms, such as catheter technology. Focusing on design innovation appropriate for smaller anatomies, the 2018 FDA guidance on pediatric medical devices urged startups and academic institutions to collaboratively develop next-generation pediatric catheterization products.
Custom-designed catheters made possible by developments in material science and 3D printing also help to save operation time and patient risk. Pediatric associations and device makers are improving physician training, increasing adoption rates, and extending long-term device performance.
Restraints:
Catheter-Related Infections, Lack of Pediatric Clinical Trial Data Continue to Limit Broader Market Expansion
In pediatric ICUs, the CDC observes that central line-associated bloodstream infections (CLABSIs) occur in 1.4 per 1,000 central line days, leading to significant morbidity, longer hospital stays, and increased care costs. This creates hesitation among physicians, especially in low-volume hospitals lacking certain pediatric infection prevention measures. Insufficient robust pediatric clinical trials are another significant limitation due to the majority of devices relying primarily on extrapolated adult data, thus compromising safety and efficacy standards for children. Only 24% of the devices utilized in pediatric populations are specifically designed for that age group, as stated by the AAP. Market regulatory differences on the global stage magnify the problem.
For instance, while there are limited pediatric pathways offered by the FDA, Asian and African markets have unclear systems for approvals of pediatric-specific devices.
Additionally, contributing to clinical distrust are frequent product recalls caused by design flaws or incompatibility with materials. Supply-side problems, such as sterilizing logistics, constrained catheter stock variance, and the shortage of pediatric interventionalists in rural settings, limit market penetration. These complex issues delay time-to-market for new entrants and limit acceptance in underdeveloped markets, thus delaying market momentum.
By Product
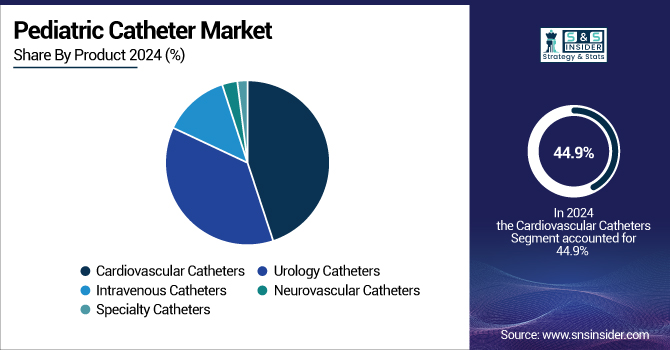
The cardiovascular catheters segment dominated the pediatric catheter market analysis with the maximum revenue share of 44.9% in 2024 due to the frequency of congenital heart defects (CHD) and the increase in demand for life-saving cardiac interventions in children and neonates. Early detection of congenital defects, advances in catheter-based therapies, and increased numbers of children's heart surgeries have contributed largely to the growing consumption of children's catheterization products in cardiology.
Further evidence is that this region is at the forefront owing to the rising utilization of less invasive cardiac surgeries, including electrophysiological testing, valve repair, and balloon angioplasty. Due to their flexibility, biocompatibility, and precise sizing fit for infants and toddlers, pediatric catheters are increasingly being selected by surgeons for these procedures. Additionally, advances in catheters, particularly designed for pediatric cardiac anatomy, have lower complication rates and improved outcomes in high-risk patients.
The urology catheters segment is likely to be the fastest-growing during 2025-2032. Increased rate of pediatric urinary tract infections, bladder dysfunctions, and neurogenic bladder associated with conditions, such as spina bifida and malformations of the spinal cord. Increasing awareness among caregivers, with improved screening for children's urine diseases, has led to early detection and treatment, thus driving the demand for specialist pediatric urine catheters and medical catheters. Additionally, the introduction of hydrophilic-coated catheters has increased patient comfort and reduced infection issues, thus supporting increased general acceptance. Particularly in long-term bladder care, the incorporation of antibacterial pediatric catheterization devices and pediatric central venous catheters in complex urological procedures is increasingly in demand.
Furthermore, anticipated segment growth is increasing non-profit and healthcare system support to fund home-based catheterization solutions. This is the most exciting product segment in the coming years, owing to the technological innovations in neonatal and infant catheters are broadening the application of urinary catheterization to the younger patient populations.
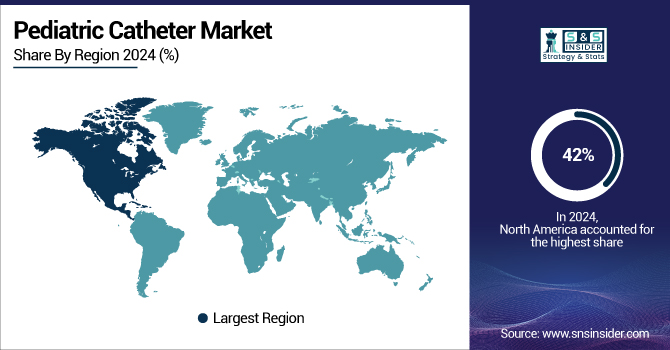
North America held leadership in the pediatric catheter market in 2024 with a 42% share, driven by advanced healthcare infrastructure, voluminous surgical demand, and effective regulatory frameworks bolstering pediatric device research. The U.S. pediatric catheter market size was valued at USD 2.79 billion in 2024 and is expected to reach USD 5.34 billion by 2032, growing at a CAGR of 8.47% over the forecast period of 2025-2032. Amplified by escalating pediatric hospital admissions, specifically of cardiovascular and urinary diseases, the United States remains the major contributor, over 65% of the regional share.
Increasing numbers of pediatric cardiovascular catheters and pediatric urinary catheters are being embraced due to more instances of congenital heart conditions and neurogenic bladder conditions. Substantial investments in new catheterization products have been made by the FDA's pediatric device programs and NIH-funded R&D. Mexico is experiencing growing demand due to greater access to healthcare in urban areas, while Canada is showing excellent absorption in minimally invasive pediatric urology.
Europe is the second fastest-growing geographical area in the pediatric catheter market due to universal healthcare facilities, early disease diagnosis, and increasing pediatric surgeries. The regional market is dominated by Germany due to its multiple specialized pediatric care institutes and academic research and development centres, particularly within the cardiovascular and neurovascular conditions. The advanced application of pediatric medical catheters in neonatal ICUs has enabled the country to be among the catheter-based pediatric interventions. Coming immediately after France and the U.K. are the countries that benefit from rising government interest in pediatric neurology and urology, and sizable pediatric research funding. Newborn and infant catheters are also assisted by the Horizon Europe program of the EU and by the European Pediatric Device Consortium. Enhanced pediatric critical care facilities and increased procedural reimbursement assisted in maintaining growth in Italy and Spain.
Asia Pacific is anticipated to be the fastest-growing market in the pediatric catheter market during 2025-2032 due to a rapidly growing pediatric population, improved access to healthcare, and growing volumes of surgery. China dominates the region due to its high incidence of congenital defects and volume of pediatric cardiac and urologic surgeries. Funded by government health programs and device clearances, significant growth in pediatric CHD procedures has generated demand for cardiovascular catheters. Backed by investments in public health infrastructure and growing awareness of pediatric urological issues, India is emerging as a principal growth center. Supported by R&D funded by the government, Japan also takes the lead in innovation, with general acceptance of less invasive catheterization products. As Australia is enhancing pediatric catheterizing practices with telemedicine and cross-border collaborations, countries including South Korea and Singapore are witnessing rapid growth in catheter-based neonatal care.
LAMEA is experiencing steady growth in the market for pediatric catheters, fostered by government initiatives and increased access to children's treatment. With Brazilian national programs addressing CHD and bladder dysfunction among children, the country has the largest market in Latin America and enjoys the widest use of pediatric medical catheters. Foremost in the Middle East with heavy public sector spending and Vision 2030 hospital expansion, Saudi Arabia pushes demand for cardiovascular catheters and pediatric urinary catheters. Increased neurogenic bladder awareness and diagnostic rates within South Africa are aiding penetration of the market. Access variations and a few approvals for pediatric devices remain problems throughout most of these countries. UAE and Qatar are rapidly modernizing pediatric ICUs to prepare themselves for future growth in innovative pediatric catheterization products, specifically neonatal and infant catheters for high-acuity care.
Get Customized Report as per Your Business Requirement - Enquiry Now
Prominent players in the pediatric catheter market include BD, Coloplast Corp, Getinge, Boston Scientific, Johnson & Johnson Private Limited, ICU Medical, Edwards Lifesciences, Medtronic, and Cook Medical.
In 2023, Medtronic's dual-lumen pediatric catheter was granted CE Mark clearance to improve circulatory assistance for pediatric patients and enhance children's treatment alternatives for severe cardiac illnesses in Europe.
In 2022, Medtronic's cardiac cryoablation catheters received approval from the FDA for their use in children, enabling targeted treatment of atrioventricular nodal reentrant tachycardia (AVNRT), which is a typical heart rhythm condition in children.
| Report Attributes | Details |
|---|---|
| Market Size in 2024 | USD 8.82 Billion |
| Market Size by 2032 | USD 18.01 Billion |
| CAGR | CAGR of 9.35% From 2025 to 2032 |
| Base Year | 2024 |
| Forecast Period | 2025-2032 |
| Historical Data | 2021-2023 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product (Cardiovascular Catheters, Urology Catheters, Intravenous Catheters, Neurovascular Catheters, and Specialty Catheters) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, Poland, Turkey, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Rest of Latin America) |
| Company Profiles | BD, Coloplast Corp, Getinge, Boston Scientific, Johnson & Johnson Private Limited, ICU Medical, Edwards Lifesciences, Medtronic, Teleflex Incorporated, and Cook Medical. |
Ans: The Pediatric Catheter market is anticipated to grow at a CAGR of 9.35% from 2025 to 2032.
Ans: The market is expected to reach USD 18.01 billion by 2032, increasing from USD 8.82 billion in 2024.
Ans: Increasing surgical volumes, healthcare infrastructure development, and public-private investment collaborations drive market growth.
Ans: Catheter-related infections, lack of pediatric clinical trial data continue to limit broader market expansion.
Ans: North America dominated the Pediatric Catheter market.
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence of Pediatric Conditions Requiring Catheterization (2024)
5.2 Pediatric Catheter Utilization Trends, by Setting (2024)
5.3 Regulatory Approvals and Pediatric Device Designations (2021–2024)
5.4 Healthcare Spending on Pediatric Catheterization, by Region and Payer (2024)
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Pediatric Catheter Market Segmentation, By Product
Chapter Overview
7.2 Cardiovascular Catheters
7.2.1 Cardiovascular Catheters Market Trends Analysis (2021-2032)
7.2.2 Cardiovascular Catheters Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Urology Catheters
7.3.1 Urology Catheters Market Trends Analysis (2021-2032)
7.3.2 Urology Catheters Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4 Intravenous Catheters
7.4.1 Intravenous Catheters Market Trends Analysis (2021-2032)
7.4.2 Intravenous Catheters Market Size Estimates and Forecasts to 2032 (USD Billion)
7.5 Neurovascular Catheters
7.5.1 Neurovascular Catheters Market Trends Analysis (2021-2032)
7.5.2 Neurovascular Catheters Market Size Estimates and Forecasts to 2032 (USD Billion)
7.6 Specialty Catheters
7.6.1 Specialty Catheters Market Trends Analysis (2021-2032)
7.6.2 Specialty Catheters Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Regional Analysis
8.1 Chapter Overview
8.2 North America
8.2.1 Trends Analysis
8.2.2 North America Pediatric Catheter Market Estimates and Forecasts, by Country (2021-2032) (USD Billion)
8.2.3 North America Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.2.4 USA
8.2.4.1 USA Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.2.5 Canada
8.2.5.1 Canada Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.2.6 Mexico
8.2.6.1 Mexico Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.3 Europe
8.3.1 Trends Analysis
8.3.2 Europe Pediatric Catheter Market Estimates and Forecasts, by Country (2021-2032) (USD Billion)
8.3.3 Europe Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.3.4 Germany
8.3.4.1 Germany Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.3.5 France
8.3.5.1 France Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.3.6 UK
8.3.6.1 UK Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.3.7 Italy
8.3.7.1 Italy Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.3.8 Spain
8.3.8.1 Spain Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.3.9 Poland
8.3.9.1 Poland Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.3.10 Turkey
8.3.10.1 Turkey Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.3.11 Rest of Europe
8.3.11.1 Rest of Europe Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.4 Asia Pacific
8.4.1 Trends Analysis
8.4.2 Asia Pacific Pediatric Catheter Market Estimates and Forecasts, by Country (2021-2032) (USD Billion)
8.4.3 Asia Pacific Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.4.4 China
8.4.4.1 China Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.4.5 India
8.4.5.1 India Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.4.6 Japan
8.4.6.1 Japan Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.4.7 South Korea
8.4.7.1 South Korea Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.4.8 Singapore
8.4.8.1 Singapore Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.4.9 Australia
8.4.9.1 Australia Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.4.10 Rest of Asia Pacific
9.4.10.1 Rest of Asia Pacific Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.5 Middle East & Africa
8.5.1 Trends Analysis
8.5.2 Middle East & Africa Pediatric Catheter Market Estimates and Forecasts, by Country (2021-2032) (USD Billion)
8.5.3 Middle East & Africa Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.5.4 UAE
8.5.4.1 UAE Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.5.5 Saudi Arabia
8.5.5.1 Saudi Arabia Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.5.6 Qatar
8.5.6.1 Qatar Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.5.7 South Africa
8.5.7.1 South Africa Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.5.8 Middle East & Africa
8.5.8.1 Middle East & Africa Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.6 Latin America
8.6.1 Trends Analysis
8.6.2 Latin America Pediatric Catheter Market Estimates and Forecasts, by Country (2021-2032) (USD Billion)
8.6.3 Latin America Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.6.4 Brazil
8.6.4.1 Brazil Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.6.5 Argentina
8.6.5.1 Argentina Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
8.6.6 Rest of Latin America
8.6.6.1 Rest of Latin America Pediatric Catheter Market Estimates and Forecasts, By Product (2021-2032) (USD Billion)
9. Company Profiles
9.1 BD
9.1.1 Company Overview
9.1.2 Financial
9.1.3 Products/ Services Offered
9.1.4 SWOT Analysis
9.2 Coloplast Corp
9.2.1 Company Overview
9.2.2 Financial
9.2.3 Products/ Services Offered
9.2.4 SWOT Analysis
9.3 Getinge
9.3.1 Company Overview
9.3.2 Financial
9.3.3 Products/ Services Offered
9.3.4 SWOT Analysis
9.4 Boston Scientific
9.4.1 Company Overview
9.4.2 Financial
9.4.3 Products/ Services Offered
9.4.4 SWOT Analysis
9.5 Johnson & Johnson Private Limited
9.5.1 Company Overview
9.5.2 Financial
9.5.3 Products/ Services Offered
9.5.4 SWOT Analysis
9.6 ICU Medical
9.6.1 Company Overview
9.6.2 Financial
9.6.3 Products/ Services Offered
9.6.4 SWOT Analysis
9.7 Edwards Lifesciences
9.7.1 Company Overview
9.7.2 Financial
9.7.3 Products/ Services Offered
9.7.4 SWOT Analysis
9.8 Medtronic
9.8.1 Company Overview
9.8.2 Financial
9.8.3 Products/ Services Offered
9.8.4 SWOT Analysis
9.9 Cook Medical
9.9.1 Company Overview
9.9.2 Financial
9.9.3 Products/ Services Offered
9.9.4 SWOT Analysis
9.10 Teleflex Incorporated
9.10.1 Company Overview
9.10.2 Financial
9.10.3 Products/ Services Offered
9.10.4 SWOT Analysis
10. Use Cases and Best Practices
11. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
By Product
Cardiovascular Catheters
Urology Catheters
Intravenous Catheters
Neurovascular Catheters
Specialty Catheters
Request for Segment Customization as per your Business Requirement: Segment Customization Request
North America
US
Canada
Mexico
Europe
Germany
France
UK
Italy
Spain
Poland
Turkey
Rest of Europe
Asia Pacific
China
India
Japan
South Korea
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
UAE
Saudi Arabia
Qatar
South Africa
Rest of Middle East & Africa
Latin America
Brazil
Argentina
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Detailed Volume Analysis
Criss-Cross segment analysis (e.g., Product X Application)
Competitive Product Benchmarking
Geographic Analysis
Additional countries in any of the regions
Customized Data Representation
Detailed analysis and profiling of additional market players