Digital Clinical Trials Market Report Scope & Overview:
Get More Information on Digital Clinical Trials Market - Request Sample Report
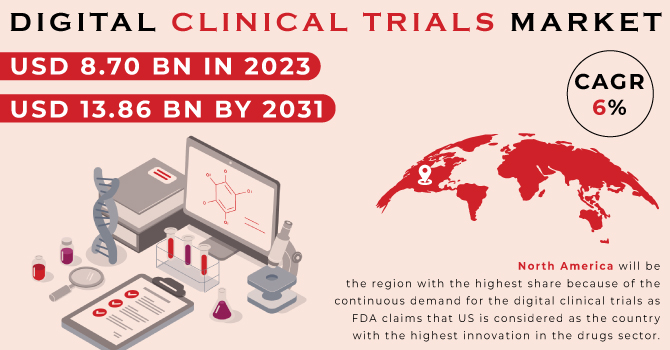
The Digital Clinical Trials Market was valued at USD 8.70 billion in 2023 and is expected to reach at USD 13.86 billion in 2031, and grow at a CAGR of 6 % over the forecast period of 2024-2031.
Digital term is familiar and is been utilized in almost every industry, with the rising demand for the digitalization because of the benefits associated with it the term digitalization will create a major difference in almost every industry, when we focus on healthcare industry there has been an immense growth in the technological advancement compared to the previous years, clinical trials are used in the healthcare industry is specifically used for the testing of the new drugs or any advancement on the humans to gain approval for that particular drug so that it can be used on a large population or targeted audience, Clinical trials in health care industry make it possible for the healthcare organization to focus on how the new invented drugs or surgical aspects works and what changes has to be made, The workshop held by NIH and NSF in 2019 regarding how digital can be implemented in the clinical trials was a positive factor for this market, FDA also plays a major part in approving the clinical trials regarding the drugs, there are certain guidelines and instructions by the FDA which every company or organization should follow in order to gain approval,
Digital Clinical trials has an opportunity to seek growth during the forecasted period digital includes the AI, data interpretation, health related data, digital retention. Digitalization in clinical trials can play a big role in terms of recording a patients data which includes heartrate, respiratory system, sleep which is an essential element when health related trails is considered, Also the fact that Covid 19 also created awareness regarding the digital clinical trials as the rules imposed by the government made it difficult to conduct human trials, Though the lack of awareness, and the adaptability amongst the stakeholders and the adaptability factor of switching from the basic standard to the digital clinical trials and the regulatory guidelines are the challenges the market might face during the forecasted period.
Impact of COVID-19:
The lockdown rules imposed by the government made it difficult for the healthcare professionals to conduct the clinical trials, every industry was adapting the digitalization as there was no alternative, Covid-19 had a great impact on the digital clinical trial market, the need for the human trials was not possible because of the Covid guidelines and also the fear of the tangible access to the corona virus gave a boom for the digital clinical trials with the help of the digital clinical trials it becomes easy in terms of patients consent, through various forms of available platforms to communicate virtually, to record the data of patients, there are various applications and software which records the data of patients and which makes it easy for the patients to record and send the data accordingly without any physical contact, well there were many arguments regarding the limitations of the digital clinical trials and many other strategies which will be implemented for the scope and usage of the digital clinical trials. Thought the covid 19 impacted the digital clinical trials where it increased the awareness of the digitalization in clinical trials and also the benefits associated with it. The most beneficial factors which got introduced during the pandemic regarding the digital clinical trials included the teleconsultation which impacted the market as a fact that in virtual clinical trial it becomes easy for the patient. The report will give you brief information about what were the exact changes which tend to happen between the phase of pre-covid and post-covid.
Digital Clinical Trials Market Dynamics
Drivers
-
Initiatives taken by the key players all around the globe to invest in the digital clinical trials post covid as pandemic created awareness for the market.
-
The increasing demand for the digital clinical trials.
Restrains
-
The fact that it is costlier than the conventional clinical trials.
Opportunity
-
Initiative taken by the government and the healthcare organizations to create awareness about the digital clinical trials
-
The benefits associated with the digital clinical trials
Challenge
-
The adaptability issue and the perceptions of stakeholders and other healthcare professionals regarding the conventional trials.
Digital Clinical Trials Market Regional Analysis:
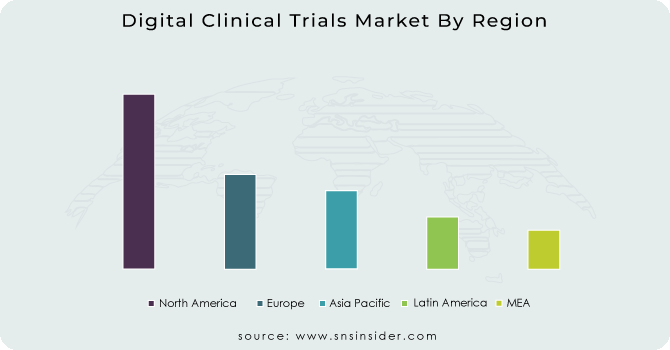
North America will be the region with the highest share because of the continuous demand for the digital clinical trials as FDA claims that US is considered as the country with the highest innovation in the drugs sector.
APAC will be the region with the highest CAGR as the urbanization and the technological advancement in the emerging and the developed nations of this region are seeking continuous growth in healthcare sector.
Need any customization research on Digital Clinical Trials Market - Enquiry Now
Key Players:
The major key players are PPD, Inc, Stignant health, Human first, CRFweb, Data Management 365, IQVIA, IBM, Deloitte and Other Players.
Recent developments
PPD Inc -The ongoing R&D to study about the digital clinical trials and how it can be implemented.
Stignant -Launch of telemedicine platform innovations to optimize remote and decentralized clinical trial operations.
These reports will give you an exact brief about what are the recent developments of all the major key players listed in the report
| Report Attributes | Details |
| Market Size in 2023 | US$ 8.70 Bn |
| Market Size by 2030 | US$ 13.86 Bn |
| CAGR | CAGR of 6% From 2024 to 2031 |
| Base Year | 2023 |
| Forecast Period | 2024-2031 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product type (Digital Wearable Devices, AI-Enabled Devices, Health App) • By Type (Decentralized/Virtual Clinical Trials, Hybrid Clinical Trials) • By Phase (Phase I, Phase II, Phase III, Phase IV) • By End User (Pharmaceutical companies, Biotechnology companies, Contract Research Organizations (CRO), Others) |
| Regional Analysis/Coverage | North America (USA, Canada, Mexico), Europe (Germany, UK, France, Italy, Spain, Netherlands, Rest of Europe), Asia-Pacific (Japan, South Korea, China, India, Australia, Rest of Asia-Pacific), The Middle East & Africa (Israel, UAE, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Rest of Latin America) |
| Company Profiles | PPD, Inc, Stignant health, Human first, CRFweb, Data Management 365, IQVIA, IBM, Deloitte, Others |
| Key Drivers | • Initiatives taken by the key players all around the globe to invest in the digital clinical trials post covid as pandemic created awareness for the market. • The increasing demand for the digital clinical trials. |
| Market Opportunities | • Initiative taken by the government and the healthcare organizations to create awareness about the digital clinical trials. • The benefits associated with the digital clinical trials |

