Healthcare Cold Chain Monitoring Market Report Scope & Overview:
The Healthcare Cold Chain Monitoring Market Size was valued at USD 2.29 billion in 2023 and is expected to reach USD 5.68 billion by 2032 and grow at a CAGR of 10.64% over the forecast period 2024-2032.
Get more information on Healthcare Cold Chain Monitoring Market - Request Sample Report
Cold chain monitoring is the use of IoT technology to continuously monitor temperature-sensitive products being transported in a "cold chain" a supply chain of perishable and/or temperature-sensitive products such as pharmaceuticals, biologics, and food& beverages. If not properly monitored, suboptimal conditions during shipping and storage may degrade the quality of these products.
MARKET DYNAMICS:
KEY DRIVERS:
-
In the healthcare industry, there is a growing need for cold chain monitoring.
-
Increased adoption of IoT technology in the healthcare industry.
RESTRAINTS:
-
Keeping temperatures stable throughout the supply chain.
OPPORTUNITIES:
-
Cold chain storage warehouse automation is in high demand.
-
Increased investment in the pharmaceutical cold chain.
CHALLENGES:
-
This will be covered under the final version of the report.
IMPACT OF COVID-19:
COVID-19 vaccines are transported safely with the help of IoT monitoring. According to the WHO, more than 40% of vaccines produced in developing countries each year are wasted due to flaws in the cold chain system. Cold chain shipping destroys 22% of temperature-sensitive biopharmaceutical products, including plasma. Excursions, or vaccines exposed to temperatures above the acceptable range. When it comes to storing or shipping vaccines or other biological items that can become inert if stored or shipped outside of a manufacturer's approved temperature or humidity range, hospitals, healthcare service providers, and logistics companies rely on refrigerated systems. This highlights the importance of cold chain monitoring systems in the healthcare industry. The ability to monitor these facilities and take corrective action in the event of a failure is critical to ensuring patient safety.
Based on the Component, the healthcare cold chain monitoring is segmented into Hardware and Software.
Based on Temperature, the healthcare cold chain monitoring is segmented into Chilled and Frozen. Certain drugs and biologics must be kept at specific temperatures, usually -25° to -10°C (-13° to 14°F), or "frozen," to remain effective. This includes, among other things, eye and ear drops, antibiotics, and injections. Cold chain providers ensure that shipments arrive at the proper temperature.
Based on Product, the healthcare cold chain monitoring is segmented into Biopharmaceuticals, Vaccines and Clinical Trial Materials. The vaccines segment held the greatest market share. Vaccines are biological products that can lose their potency if exposed to high temperatures or freezing temperatures.
Based on End User, the healthcare cold chain monitoring is segmented into Hospital & Clinics, Biopharmaceutical Companies and Research Institutes. Biopharmaceutical companies work with complex medicinal products made from living cells and/or organisms that have undergone advanced biotechnological processes.
KEY MARKET SEGMENTATION:
BY PRODUCT
-
Biopharmaceuticals
-
Vaccines
-
Clinical Trial Materials
BY COMPONENT
-
Hardware
-
Software
BY TEMPERATURE
-
Chilled
-
Frozen
BY END USER
-
Hospital & Clinics
-
Biopharmaceutical Companies
-
Research Institutes
REGIONAL ANALYSIS:
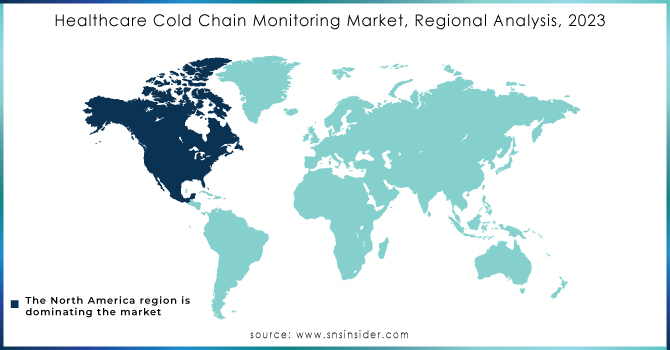
The market for healthcare cold chain monitoring has been researched in Asia-Pacific, North America, Europe, and the Rest of the World. North America held the largest market share. This can be attributed to the growing need for customised storage and transportation to ensure the effectiveness of healthcare products, as well as the region's growing emphasis on improving business agility.
The adoption of cold chain monitoring solutions by the midsize healthcare industry is expected to drive growth in the European healthcare cold chain monitoring market. The country's leading global cold chain monitoring innovator is constantly working to introduce new technologies.
Because of favourable government policies, the Chinese healthcare sector has expanded rapidly. China is an important player in the Asia-Pacific market due to its large population and increasing demand for cloud solutions by biopharmaceutical companies.
Need any customization research on Healthcare Cold Chain Monitoring Market - Enquiry Now
REGIONAL COVERAGE:
-
North America
-
USA
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
The Netherlands
-
Rest of Europe
-
-
Asia-Pacific
-
Japan
-
south Korea
-
China
-
India
-
Australia
-
Rest of Asia-Pacific
-
-
The Middle East & Africa
-
Israel
-
UAE
-
South Africa
-
Rest of Middle East & Africa
-
-
Latin America
-
Brazil
-
Argentina
-
Rest of Latin America
-
KEY PLAYERS:
The key players in the healthcare cold chain monitoring market are Carrier, Berlinger, Testo SE, Signatrol, Temptime Corporation, Monnit Corporation, Emerson Electric, KGaA, Haier Biomedical and Cold Chain Technologies and other players.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 2.29 Billion |
| Market Size by 2032 | US$ 5.68 Billion |
| CAGR |
CAGR of 10.64% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product (Biopharmaceuticals, Vaccines, Clinical Trial Materials) • By Component (Hardware, Software) • By Temperature (Chilled, Frozen) • By End User (Hospital & Clinics, Biopharmaceutical Companies, Research Institutes) |
| Regional Analysis/Coverage | North America (USA, Canada, Mexico), Europe (Germany, UK, France, Italy, Spain, Netherlands, Rest of Europe), Asia-Pacific (Japan, South Korea, China, India, Australia, Rest of Asia-Pacific), The Middle East & Africa (Israel, UAE, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Rest of Latin America) |
| Company Profiles | Carrier, Berlinger, Testo SE, Signatrol, Temptime Corporation, Monnit Corporation, Emerson Electric, KGaA, Haier Biomedical and Cold Chain Technologies. |
| Key Drivers | • In the healthcare industry, there is a growing need for cold chain monitoring. • Increased adoption of IoT technology in the healthcare industry. |
| Restraints | • Keeping temperatures stable throughout the supply chain. |

