Huntington’s Disease Treatment Market Report Scope & Overview
The Huntington’s Disease Treatment market size was USD 445.90 million in 2023 and is expected to reach USD 2670.13 million by 2032 and grow at a CAGR of 22.00% over the forecast period of 2024-2032. This report provides comprehensive insights into the Huntington’s disease treatment market, including detailed prevalence and incidence statistics across key regions and age groups. The report showcases the recent market trends in the diagnosed versus undiagnosed cases and the surging number of genetic carriers. The study also examines the growing clinical pipeline, with a strong focus on the ongoing trials and drug development stages. The surged burden of healthcare and treatment costs are analyzed in the report to assess the economic impact. Regulatory insights, such as approval timelines and orphan drug designations, are also covered in the report.
Additionally, the report explores the current trends in genetic screening uptake and patient support initiatives. Collectively, these insights also provide a well-rounded view of the market beyond standard sizing and segmentation.
The U.S. held the largest share in the Huntington’s disease treatment market in 2023, valued at USD 140.98 Million, and is projected to reach USD 862.29 Million by 2032, growing at an impressive CAGR of 22.29% during the forecast period. The dominance of the market is propelled by many major factors, such as the high Huntington’s disease prevalence in the U.S., ranging between 5 and 10 cases per 100,000 people globally. The country also benefits from strong diagnostic capabilities, advanced healthcare infrastructure, and global availability of genetic testing, further enabling for earlier and accurate detection.
Although, the U.S. has a highly active pipeline of drug development backed by substantial investments from both the private and public. Many regulatory incentives, including fast-track and orphan drug designation approvals by the U.S. FDA, further augmenting the therapeutic innovation. In addition, a well-established network of patient advocacy groups and clinical research institutions ensures large awareness, easy access to emerging treatments, and early intervention, further reinforcing the leading position of the U.S. in the global market.
Market Dynamics:
Drivers
-
Rising Prevalence of Huntington’s Disease Drives Treatment Demand Globally
The Huntington’s disease treatment market growth is substantially driven by the surging prevalence of the Huntington’s disease and growing advancements in the genetic testing technologies. The accurate and early diagnosis via genetic screening has enhanced the disease management and treatment initiation, thus increasing the patient pool. In the developed regions, enhanced family history tracking, awareness campaigns, and high accessibility to genetic counseling, are all contributing to a surge in the diagnosis rates. However, investment in genetic therapies and research collaborations by the key players, such as Wave Life Sciences and Ionis Pharmaceuticals have further boosted the growth of the market. This surging burden of the disease, incorporate with the technological advances in disease diagnostics and a proactive healthcare system, continues to aid the Huntington’s disease treatment market growth.
Restraints:
-
High Treatment Costs and Limited Advanced Therapies’ Affordability Hinders Market Expansion Globally
One of the key restraints impeding the Huntington’s disease treatment market growth is the high cost of the therapies available and limited affordability in the low-income nations globally. Advanced therapies including antisense oligonucleotide and gene-silencing treatments, which generally cost tens of thousands of dollars per patient annually. The long-term disease management require continuous support, symptomatic treatment, and rehabilitation contributing to a high economic burden. In several regions, the lack of reimbursement frameworks in the public healthcare systems for rare diseases, such as Huntington’s disease, which makes treatment inaccessible to a substantial portion of the global population. Even in developed regions, the insurance coverage for emerging therapies is still uncertain, further hampering the patient access. As a result, even after the growing awareness and clinical advancements, the adoption rates of such treatments in the under-resourced nations remain low, curbing the overall global market penetration and negatively affecting the equitable treatment distribution across various regions.
Opportunities:
-
Emergence of Gene-Silencing and Disease-Modifying Therapies Offers Strong Growth Opportunities for Key Market Players
Significant growth opportunities in the Huntington’s disease treatment market are expected to arise due to the rising development of gene-silencing and disease-modifying therapies globally. Traditional treatments are initially focused on managing symptoms; however, the recent innovations are targeting the root genetic causes of Huntington’s disease. Several key players, such as Novartis, Roche, and Wave Life Sciences are actively engaged in the clinical trials to launch such therapies in the market. The burgeoning number of regulatory designations, including the Orphan Drug and Fast Track status also boosts the drug approvals and R&D efforts. These innovations have the potential to reduce the disease progression that can completely transform the treatment landscape of the disease. With the growing number of investments from the government and biotech firms, and strong pipeline of candidates, the launch of new and effective gene-targeting therapies can not only increase the life expectancy but also improve the quality of life for patients globally.
Challenges:
-
Lack of Curative Therapies and Limited Disease Progression’s Understanding Pose Substantial Challenges for Market Augmentation
A substantial challenge impacting the growth of the Huntington’s disease treatment market is the lack of disease’s complex progression understand and limited availability of curative therapies. Huntington’s Disease affects different neurological functions, which are progressing differently across patients, further complicating both diagnosis and treatment planning. In spite of the advancements in genetic testing, along with symptom severity, prediction of the exact onset age of onset, and progression speed remains difficult.
However, currently, there are no approved existing therapy, which fully curb or reverse the neurodegeneration related with the disease. The current medications only offer symptomatic relief, and several experimental drugs have not been able to generate results in the late-stage clinical trials owing to the safety concerns or limited efficacy. This scientific gap not only extends patient suffering but also have a major hurdle for pharmaceutical companies that are focusing on the development of breakthrough treatments, further decreasing the speed of clinical success and innovation in the field.
Segmentation Analysis
By Treatment
Based on treatment, the market is bifurcated into symptomatic treatment and disease-modifying therapies. The symptomatic treatment held the largest Huntington’s disease treatment market share of around 78% in 2023. The lack of approved disease-modifying therapies and the growing urgent need for managing the large range of debilitating symptoms related to the condition drive the segment’s expansion in the market. Huntington’s disease grows via progressive motor dysfunction, psychiatric disturbances, and cognitive decline, all of which substantially affecting the patient’s quality of life. As no cure for the disease currently exists, the treatment strategies are initially focused on reducing these symptoms by utilizing medications including antidepressants, antipsychotics, and mood stabilizers.
Additionally, supportive interventions, such as occupational therapy and physiotherapy are commonly adopted to improve patients’ daily functioning. The regulatory approval, accessibility, and established efficacy of these treatments have contributed to their large adoption in the clinical practices globally. As a result, symptomatic treatment remains the foundation of patient care, further boosting the segment in the market.
By End-Use Industry
On the basis of end-use industry, the market is divided into hospital pharmacy, retail pharmacy, and e-commerce. In 2023, the hospital pharmacy segment dominated the market holding a revenue share of around 57%. The segment’s expansion is propelled by the specialized and complex nature of managing the disease that generally requires coordinated care and advanced or high-cost therapies prescription. Patients suffering from Huntington’s disease frequently witness a range of serious psychiatric and neurological symptoms, which demand close monitoring, multidisciplinary support, and dose adjustments, which are easily available in the hospital settings.
Although, hospital pharmacies are considered to be the initial dispensing points for the latest or investigational treatments, which include gene-silencing therapies or drugs under concerned programs. These facilities also confirm compliance with the strict and regulatory, especially for the orphan drugs. In addition, the growing availability of neurologists and specialized care teams in hospitals makes hospital pharmacy, a preferred setting to initiate and manage treatment, further strengthening the dominance of hospital pharmacies in the market.
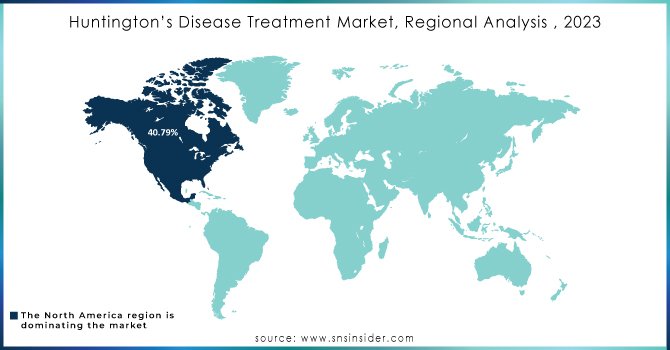
Regional Analysis
In 2023, North America was the dominating region in the market holding a revenue share of approximately. The region’s growth is driven by a combination of advanced healthcare infrastructure, strong research and development investments, and high disease prevalence. North America, particularly the U.S., has recorded some of the highest diagnosis rates across the globe, with approximately 10 cases per 100,000 people, propelled by the wide access to the early screening and genetic testing programs. North America is also benefited from a strong network of academic research centers and biopharmaceutical companies, which are actively engaged in the development of novel treatments, such as disease modifying drugs and gene therapies. Furthermore, several supportive regulatory frameworks, including the U.S. FDA’s Orphan Drug Designation and Fast Track programs, have boosted the drug development and approvals in the region. Strong insurance coverage, high healthcare spending, and well-established established treatment protocols further improve patients’ access to advanced therapies, setting North America’s leadership in the global market.
Asia Pacific held a substantial market share in the Huntington’s disease treatment market owing to its large population base, along with surging rare genetic disorders awareness, and enhancing healthcare infrastructure across multiple emerging economies. Nations including South Korea, China, and Japan, have been experiencing burgeoning efforts in the early diagnosis and genetic testing, backed by several government health initiatives and the neurology-focused healthcare services expansion. In addition, several international partnerships with the global pharmaceutical firms are allowing for faster access to innovative clinical trials and innovative treatments in the region. The increasing availability of specialized care in the urban centers, integrated with the growing healthcare expenditure, is further boosting the demand for Huntington’s disease treatment globally.
However, rising patient advocacy efforts and supportive regulatory reforms are encouraging earlier diagnosis and treatment adoption, making Asia Pacific an influential and rapidly emerging market globally.
Do You Need any Customization Research on Huntington’s Disease Treatment Market - Enquire Now
Key Players
-
Neurocrine Biosciences (Ingrezza, Valbenazine)
-
NeuExcell Therapeutics Inc (NEU-CH16, NEU-CH20)
-
H. Lundbeck A/S (Xenazine, Austedo)
-
Teva Pharmaceutical Industries Ltd (Austedo, Azilect)
-
Bausch Health Companies Inc. (Tetrabenazine, Aplenzin)
-
Hetero (Tetrabenazine, Haloperidol)
-
Lupin (Risperidone, Haloperidol)
-
Hikma Pharmaceuticals PLC (Tetrabenazine, Quetiapine)
-
Dr. Reddy’s Laboratories Ltd. (Tetrabenazine, Risperidone)
-
Sun Pharmaceutical Industries Ltd. (Tetrabenazine, Olanzapine)
-
Novartis AG (Exelon, Tegretol)
-
Pfizer Inc. (Zoloft, Geodon)
-
Roche Holding AG (Tominersen, Madopar)
-
Ionis Pharmaceuticals, Inc. (IONIS-HTTRx, BIIB067)
-
Wave Life Sciences (WVE-003, WVE-120101)
-
Sage Therapeutics (SAGE-718, SAGE-324)
-
Azevan Pharmaceuticals (SRX246, SRX251)
-
Voyager Therapeutics (VY-HTT01, VY-AADC)
-
uniQure N.V. (AMT-130, Glybera)
-
PTC Therapeutics, Inc. (PTC518, Emflaza)
Recent Development:
-
In December 2024, PTC Therapeutics signed a licensing deal with Novartis valued at up to USD 2.9 billion for PTC518, an experimental drug targeting Huntington’s disease. The drug has demonstrated potential in lowering mutant Huntingtin protein levels. Novartis will lead the drug’s development, manufacturing, and commercialization.
-
In May 2024, Teva received U.S. FDA approval for its extended-release Austedo (deutetrabenazine) tablets as a once-daily treatment for chorea linked to Huntington's disease. This milestone is anticipated to improve patient adherence. It also strengthens Teva's presence in the Huntington’s disease treatment market.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 445.90 Million |
| Market Size by 2032 | USD 2670.13 Million |
| CAGR | CAGR of 22.00% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Treatment (Symptomatic Treatment, Disease-modifying Therapies) • By End-Use Industry (Hospital Pharmacy, Retail Pharmacy, E-commerce) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Neurocrine Biosciences, NeuExcell Therapeutics Inc, H. Lundbeck A/S, Teva Pharmaceutical Industries Ltd, Bausch Health Companies Inc., Hetero, Lupin, Hikma Pharmaceuticals PLC, Dr. Reddy’s Laboratories Ltd., Sun Pharmaceutical Industries Ltd, Novartis AG, Pfizer Inc., Roche Holding AG, Ionis Pharmaceuticals Inc., Wave Life Sciences, Sage Therapeutics, Azevan Pharmaceuticals, Voyager Therapeutics, uniQure N.V., PTC Therapeutics Inc. |

