Leukapheresis Market Size:
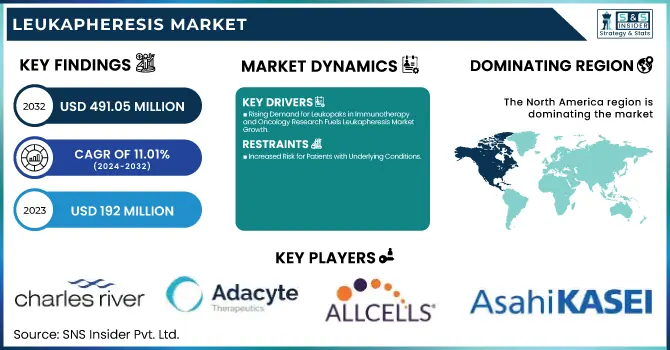
The Leukapheresis Market Size was valued at USD 192 Million in 2023 and is expected to reach USD 491.05 Million by 2032 and grow at a CAGR of 11.01% over the forecast period 2024-2032.
Get More Information on Leukapheresis Market - Request Sample Report
The leukapheresis market is robustly growing because of the rising incidence of blood-related disorders and autoimmune diseases, technologies related to leukapheresis advanced in leaps and bounds, and demand for personalized medicine has increased widely. Blood disorders such as leukemia and lymphoma and autoimmune diseases such as rheumatoid arthritis and multiple sclerosis largely contribute to the growth of the market. According to The Leukemia & Lymphoma Society, in the United States, there are about 184,720 new cases of leukemia, lymphoma, or myeloma to be diagnosed in 2023. A Lancet study of May 2023 suggested that autoimmune conditions affect 1 in 10 and have growing prevalence with multifaceted issues, environmental, resulting in cases such as rheumatoid arthritis and type 1 diabetes. These statistics show an increasing number of patients demanding leukapheresis procedures.
Technological advancements propel the market growth further with new systems including automated devices, continuous flow centrifugation, and closed system devices that increase efficiency, safety, and yields of cell collection. For example, Terumo BCT's Spectra Optia Apheresis System in which the Spectra Optia is a mononuclear cell collection device that increases the safety and efficiency of cell therapy applications. Against this background, the American Society for Apheresis and other similar bodies established guidelines in October 2022 that updated leukapheresis protocols to optimize procedure quality across age ranges and research settings creating an avenue for industry development.
Other factors include a growing focus on personalized medicine, particularly for cell therapies such as CAR-T cell therapy and stem cell transplants, which are dependent on high-quality leukapheresis products for success. The highly complex regulation in the industry of leukapheresis means that high barriers to entry exist mainly in the form of very stringent requirements regarding matters such as safety and efficacy. However, the latter is likely to be stimulated by increasing demand due to growing conditions of blood and immune disorders. Companies like Haemonetics Corporation expand their global footprint through strategic partnering to penetrate emerging markets and support moderate regional growth. Despite relatively low levels of merger and acquisition activity, these factors collectively place the leukapheresis market to accelerate growth in the future.
Market Dynamics
Drivers
-
Rising Demand for Leukopaks in Immunotherapy and Oncology Research Fuels Leukapheresis Market Growth
The most significant driver of the leukapheresis market is the increasing demand for leukopaks in clinical research. Leukopaks are products of leukapheresis, rich in various types of immune cells such as lymphocytes, monocytes, and dendritic cells, and are used very critically in oncology, immunotherapy, and cell biology research. Due to their versatility and high concentration of immune cells, leukopaks are highly essential in a wide range of therapeutic and research applications, particularly in studies that require human cells.
With increasing activities from pharmaceutical and biotech companies towards cell-based immunotherapies, such as CAR-T therapy against leukemia, the demand for leukopaks is on the rise. CAR-T therapy utilizing T-cells derived from leukopaks for the generation of genetically modified chimeric antigen receptor T-cells has proved incredibly effective in the treatment of B-cell malignancies and multiple myeloma. As of today, nearly 300 pharmaceutical companies are actively involved in developing CAR-T therapy. ClinicalTrials.gov accounts for ongoing 316 CAR-T cell-related clinical trials within the United States up to August 2023, a sign that the interest in this type of therapy is growing as well as promising. Six CAR-T cell therapies have been approved through April 2023, owing to this groundbreaking efficacy. Nevertheless, these treatments carry risks like cytokine release syndrome and immune effector cell-associated neurotoxicity, which further reinforces the necessity for new methods while mitigating the risk in that particular segment. The need for such highly advanced immunotherapies and other research studies is likely to drive the leukapheresis market significantly.
Restraints
-
Risk of Complications and Adverse Effects
Therapeutic leukapheresis is associated with various complications, including decreased white blood cell (WBC) count, adverse reactions to anticoagulants, hypocalcemia, and potential risks of RBC or platelet loss. Such adverse effects can lead to dizziness, pain at the venipuncture site, and more severe complications in vulnerable patients, limiting the procedure's acceptance and usage.
-
Increased Risk for Patients with Underlying Conditions:
For donors with anemia, thrombocytopenia, or other underlying health issues, therapeutic leukapheresis poses significant risks, including pulmonary leukostasis and acute renal failure. These risks heighten concerns and serve as a deterrent in the market, particularly in cases where the potential for immune response complications is high.
Key Segmentation Analysis
By Product
The leukapheresis devices segment dominated the 2023 market and held the largest revenue share of 76.4%. The segment is further divided into centrifugation-based devices and membrane filtration-based devices. In centrifugation-based devices, there is going to be increased demand with the escalating level of blood disorders, such as leukemia and lymphoma, and more and more stem cell transplants to be done with technology speeding up advancement and efficiency and automation capabilities improving. Membrane filtration-based devices are also in demand due to increasing cases of autoimmune diseases and chronic illness, with the need for therapeutic apheresis and for depleting or recovering certain cell populations like white blood cells. In January 2023, the company StemExpress launched CellsExpress, which is meant to streamline research by providing bulk, customized isolated cell types from high-quality Leukopaks, thereby speeding up R&D processes. Further, these new product launches are anticipated to fuel up the demand for leukapheresis devices in the forecast period.
Leukapheresis disposables are expected to witness the fastest growth over the forecast period. This is due to enhanced procedure volume, greater awareness of blood component separation techniques, and stringent regulatory standards that focus more on patient safety. With the rise in the patient population with hematologic disorders and solid tumors, the number of procedures is also increasing due to the demand for disposables used in leukapheresis. For example, Haemonetics Corporation has a set of disposable kits that are compatible with the MCS+ system offered by the company to ensure effective cell collection and processing. The trend is likely to contribute to this market's growth during the forecast period.
By Application
Research applications accounted for the largest share of the market in 2023, with a revenue share of 67.9%. The growth in the application area is mainly because of the increasing interest in the development of novel therapies for diseases such as cancer and autoimmune disorders. Such research applications are expected to fuel growth in demand for leukapheresis procedures during the next forecast period as cells, such as T cells and stem cells, are isolated to further their study and therapeutic uses. For instance, the study in the journal Cancers, published in December 2023, highlighted the fact that emergency leukapheresis might improve the outcome and reduce the risk of early mortality among patients with acute myeloid leukemia and FLT3-ITD mutations more than leukocytosis.
The therapeutic application segment is set to grow at a compound annual growth rate of 11.51% over the forecast period. This growth is due to the increasing adoption of cell-based therapies and personalized medicine. A news article by Transfusion Medicine and Hemotherapy published in August 2022, stated that even if the patient population is smaller, therapeutic leukapheresis remains safe, effective, and available in the management of specific conditions.
Regional Analysis
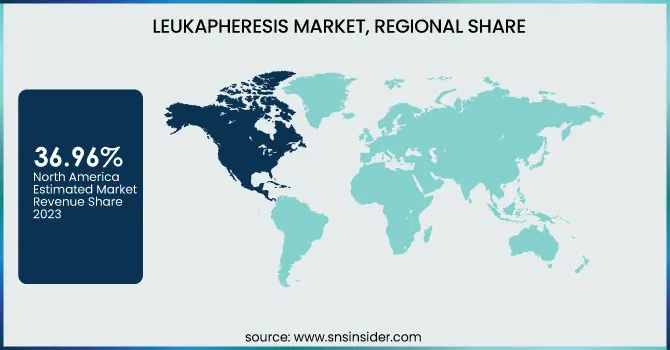
In 2023, North America dominated the leukapheresis market with a revenue share of 36.96% due to its sophisticated healthcare infrastructure, high healthcare expenditure, and increasing prevalence of diseases like cancer and autoimmune disorders. The intense research and development activities coupled with the presence of major pharmaceutical and biotechnology companies with their interest in developing innovative treatments account for a significant contribution to the growth of the market in the region. Over the next few years, such factors are likely to sustain their momentum in this market. Improvements in healthcare technology and increased healthcare expenses will bear growth to this U.S. leukapheresis market. The leukapheresis procedure will gain wider acceptance by hospitals and other care settings, thus catalyzing further growth in the coming years.
The market is expected to grow rapidly in Europe as it involves greater expenditure on healthcare and improves awareness of therapeutic innovation. The technology of leukapheresis is going to be rapidly developed and adopted with collaborations between research institutions, pharmaceutical firms, and healthcare providers. The demand for leukapheresis is rising in the UK concerning incidence rates of blood cancers and autoimmune diseases. France will also experience high market growth, as research and development with positive government regulations regarding biotechnology and healthcare will continue. German segment is expected to have the fastest growth in that region primarily due to increased investments in new leukapheresis techniques and personalized medicine.
The Asia Pacific region is the fastest-growing region in the global leukapheresis market, with a CAGR of 12.2% from 2024 to 2032. Developing healthcare expenses, better infrastructure, and heightened awareness of advanced treatments are expected to fuel growth in this region. Amongst such countries, China, Japan, and India are going to experience strong growth, with investment in medical infrastructures and a higher prevalence of chronic diseases.
Need Any Customization Research On Leukapheresis Market - Inquiry Now
Key Players
-
Charles River Laboratories International, Inc. – (Leukopaks)
-
Adacyte Therapeutics – (Leukopaks)
-
AllCells, LLC – (Fresh Leukopaks, Frozen Leukopaks)
-
Asahi Kasei Medical – (Cellsorba)
-
Haemonetics Corporation – (NexSys PCS, MCS+, PCS2)
-
Macopharma – (Leukokit)
-
Cerus Corporation – (INTERCEPT Blood System)
-
SB-Kawasumi Laboratories, Inc. – (Leukapheresis Kits)
-
StemExpress, LLC – (Clinical Grade Leukopaks)
-
Lonza Group AG – (Lovo Cell Processing System)
Recent Developments
-
In March 2024, the FDA approved Bristol Myers Squibb’s Breyanzi, an innovative CAR T cell therapy for adult patients with specific types of leukemia and lymphoma. The treatment process begins with leukapheresis, where patients' white blood cells are collected to create the therapy.
-
In August 2023, the FDA approved the Reveos Automated Whole Blood Processing System by Terumo Blood and Cell Technologies. This system improves the efficiency of processing whole blood into white blood cells and other components, supporting efforts to increase the U.S. blood supply.
-
In May 2023, Akadeum Life Sciences launched a new product line for cell therapy research, advancing their BACS Microbubble technology. This innovation enables straightforward isolation of T cells and PBMCs from leukapheresis materials, eliminating the need for traditional lysis or centrifugation steps.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 192 Million |
| Market Size by 2032 | US$ 491.05 Million |
| CAGR | CAGR of 11.01% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | •By Product [Leukapheresis Devices (Centrifugal Devices, Membrane Separators), Leukapheresis Disposables] •By Application [Research Application (Cancer Research, Immunology Research, Others), Therapeutics Application (Hematologic Disorders, Autoimmune Diseases, Others)] •By End-Use (Blood Centers, Academic & Research Institutes, Pharmaceutical & Biotechnology Companies, Hospitals & Clinics) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Charles River Laboratories International, Inc., Adacyte Therapeutics, AllCells, LLC, Asahi Kasei Medical, Haemonetics Corporation, Macopharma, Cerus Corporation, SB-Kawasumi Laboratories, Inc., StemExpress, LLC, Lonza Group AG |
| Key Drivers | • Rising Demand for Leukopaks in Immunotherapy and Oncology Research Fuels Leukapheresis Market Growth |
| Restraints | • Risk of Complications and Adverse Effects • Increased Risk for Patients with Underlying Conditions |

