Allergy Immunotherapy Market Size Analysis:
The Allergy Immunotherapy Market was valued at USD 1.77 billion in 2023 and is expected to reach USD 4.01 billion by 2032, growing at a CAGR of 9.48% from 2024-2032.

To Get more information on Allergy Immunotherapy Market - Request Free Sample Report
This report provides an analytical advantage by providing in-depth statistical information in various aspects of the Allergy Immunotherapy Market. It contains detailed information on the prevalence and incidence of allergic diseases, giving a clear picture of disease burden among global populations. The report also addresses regional prescription patterns for allergy immunotherapy, identifying treatment adoption differences. It also breaks down product use patterns by treatment type—subcutaneous and sublingual immunotherapy. Such data-driven trends allow stakeholders to measure long-term changes in clinical preferences and therapy delivery modes and provide a highly data-intensive tool beyond traditional growth indicators.
The U.S Allergy Immunotherapy Market was valued at USD 0.52 billion in 2023 and is expected to reach USD 1.14 billion by 2032, growing at a CAGR of 9.19% from 2024-2032. The United States is in a leading position in the North American allergy immunotherapy market because allergic conditions like allergic rhinitis and asthma have a high prevalence rate, and there exists a strong healthcare infrastructure and powerful diagnostic facilities. Moreover, key market players and existing clinical research efforts significantly contribute to the leadership position.
Allergy Immunotherapy Market Dynamics
Drivers
-
The growing worldwide burden of allergic diseases like allergic rhinitis, asthma, and food allergy is a major growth driver for the allergy immunotherapy market.
As per the World Allergy Organization, as much as 30–40% of the world's population now has at least one allergic disorder, and allergic rhinitis alone affects an estimated 500 million individuals worldwide. This consistent increase has triggered a heightened demand for long-term and disease-altering treatments such as subcutaneous and sublingual immunotherapy. To address this, market leaders are increasing treatment portfolios. For example, ALK-Abelló has launched its Allergy+ strategy to address new allergic conditions, such as food allergy and anaphylaxis. This increase in disease incidence, combined with changing therapeutic development, is creating robust market traction and dictating future treatment paradigms in major geographies.
-
Favorable Regulatory Approvals and Expanding Product Pipelines are propelling the market growth.
Regulatory agencies like the FDA and EMA have also grown more favorable to allergy immunotherapy, accelerating approval for new products and increasing indications for established treatments. Recent approvals—like DBV Technologies' advances on Viaskin Peanut and Aimmune Therapeutics' FDA-approved Palforzia—illustrate an upswing toward embracing standardized protocols for immunotherapy. Not only do these developments increase treatment choice, but they also enhance patient trust in immunotherapy treatments. In addition, firms are making significant investments in clinical trials to advance next-generation biologics and oral immunotherapies. With positive regulatory environments and efficient clinical pathways, the allergy immunotherapy market is enjoying accelerated time-to-market for novel therapies to drive both innovation and competition. This trend is crucial to addressing the growing unmet needs across pediatric and adult allergy treatment segments.
Restraint
-
High cost of treatment and limited reimbursement coverage are restraining the market growth.
Among the principal constraints to the allergy immunotherapy market is the expense related to long-course treatment regimens, especially subcutaneous and sublingual therapy. Immunotherapy tends to need extended administration—anywhere from months to years—due to which it is less likely to be utilized by patients without sufficient insurance coverage. The reality is compounded even more in the low- and middle-income economies, where there are no priority or reimbursement facilities available for such therapies under the public healthcare programs. Furthermore, in markets such as Asia and Latin America, out-of-pocket spending is still a major impediment, deterring patient adoption. Even in developed economies, reimbursement schemes can fail to cover the entire course or range of available treatments. This cost cuts down on adherence and caps market penetration. As a result, even with clinical effectiveness, affordability remains a limiting factor to widespread adoption of allergy immunotherapy, particularly in underprivileged communities.
Opportunities
-
The large potential in the allergy immunotherapy market is growing in emerging economies and the children's segment.
Asia Pacific countries, Latin American nations, and regions of the Middle East and Africa are observing an increase in allergic diseases resulting from urbanization, environmental pollution, and modified lifestyles. Such regions are untouched as a result of limited accessibility to high-value treatments. Spreading awareness, better healthcare infrastructure, and rising investments from international drug firms are paving the way for higher penetration of allergy immunotherapy. Moreover, the pediatric demographic is being widely recognized as a promising target segment for early treatment. Advances in child-friendly formulations like oral drops and oral disintegrating tablets are fueling adoption growth. These factors open up prosperous growth opportunities for businesses that seek to extend their coverage and diversify the patient base.
Challenges
-
One of the most important issues in the allergy immunotherapy business is the paucity of standard allergens and the demand for region-wise customization.
Geographies differ by climate, plants, and ways of life, leading to geographical variation in allergens, requiring tailored immunotherapies to be developed. Regional differences impose obstacles in large-scale manufacturing, regulatory clearances, and affordability through distribution. Furthermore, a lack of global-standard extracts for allergens impacts uniformity in treatments and clinical effects. For example, allergens in pollen are quite different between Europe and Asia, necessitating different product composition and clinical trial requirements. These scientific and regulatory challenges extend product development timelines and drive up manufacturing costs. Such a challenge to overcome requires widespread regional research, cooperation with regional healthcare authorities, and adaptable manufacturing capabilities—processes not available to all in the market, nor can all of them utilize them effectively.
Allergy Immunotherapy Market Segmentation Analysis
By Treatment Type
In 2023, the subcutaneous immunotherapy (SCIT) segment dominated the allergy immunotherapy market with a 65.14% market share as a result of its established clinical effectiveness, physician comfort, and robust safety record. SCIT is commonly known as "allergy shots," and it has been the traditional method for managing a broad variety of allergic disorders like allergic rhinitis, asthma, and insect venom allergy. Its capacity to achieve long-term desensitization and symptom alleviation with a fairly well-standardized dosage regimen has made it the allergists' first choice. Additionally, the convenience of available customized allergen extracts and insurance reimbursement in countries such as North America and Europe also increased its dominance in the market. Its formal administration in clinic settings also enables easy monitoring, which has increased its believability among medical practitioners and patients.
Sublingual immunotherapy (SLIT) segment is expected to see the fastest growth with 9.62% CAGR over the forecast period in the allergy immunotherapy market during the forecast period, fueled by its patient-friendly route of administration and increasing product approvals. SLIT, which comes in the form of tablets or drops, provides a needle-free option that can be self-administered at home, greatly enhancing patient compliance and convenience. This form of treatment is particularly attractive for pediatric and geriatric groups who are reluctant or inappropriate candidates for standard injections. Recent clinical trials have shown equal efficacy and better safety profiles with SLIT, and its growing regulatory approval in Europe, the U.S., and Asia. Moreover, the pharmaceutical industry is investing in broadening the portfolio of SLIT-based products targeting allergens, including grass, ragweed, and dust mites, which is contributing to its increased adoption worldwide.
By Type
The tablets segment dominated the allergy immunotherapy market with a 55.17% market share in 2023, attributed to its convenience, patient compliance, and approvals in the key markets. Allergy immunotherapy tablets sublingual immunotherapy (SLIT) tablets in particular are poised for self-administration at home, reducing the burdensome frequent clinic visits of subcutaneous immunotherapy (SCIT). This self-administration benefit contributes substantially towards improved compliance, particularly with children and the elderly. In addition, major drug manufacturers have developed a variety of standardized tablets for treating common allergens like grass pollen, ragweed, and house dust mites. Brands such as GRAZAX and Oralair have been well-received in Europe and North America based on their established efficacy and safety profiles. The widespread accessibility, expanding doctor confidence, and rising demand for non-surgical treatments have further entrenched the dominance of tablet treatments in the field of allergy immunotherapy.
By Allergy Type
In 2023, the allergic rhinitis segment dominated the allergy immunotherapy market with a 72.16% market share, based largely on its high global prevalence and huge impact on the quality of life in patients. Allergic rhinitis impacts approximately 10–30% of the world population, although significantly higher figures have been noted in industrialized countries because of heightened exposure to airborne allergens like pollen, dust mites, and pet dander. Being a chronic and frequently underdiagnosed condition, allergic rhinitis results in significant healthcare visits and lost productivity. Immunotherapy is especially beneficial for moderate-to-severe cases that do not respond favorably to standard antihistamines or corticosteroids. The presence of specially designed subcutaneous and sublingual treatments that target prevalent allergens responsible for allergic rhinitis has further solidified the dominance of the segment, leading it to be a prime focus of research, development, and clinical use within the realm of allergy immunotherapy.
By Distribution Channel
In 2023, the hospital pharmacy segment dominated the allergy immunotherapy market with a 40.11% market share, because of the clinical sophistication of treatment administration and the need for patient monitoring. Several allergy immunotherapy products—especially subcutaneous immunotherapy (SCIT)—need professional management for initial dosing and dose incrementation, usually requiring administration in a hospital or specialty care environment. Hospital pharmacies are responsible for ensuring the safe storage, preparation, and dispensing of allergen extracts and associated biologics according to strict regulatory guidelines. Integrated hospital systems also enable coordinated care, enabling allergists and immunologists to prescribe and track patient outcomes efficiently. These conditions have supported hospital pharmacies as the main vehicle for dispensing products of allergy immunotherapy, particularly for moderate to severe allergies demanding long-term adherence and physician supervision.
The online pharmacies segment is anticipated to experience the fastest growth over the forecast period based on the growing need for convenience, affordability, and access to maintenance allergy immunotherapy treatments—most notably sublingual immunotherapy (SLIT) tablets and drops. With increasing popularity of SLIT because of ease of administration and good safety profile, patients are increasingly preferring home-based therapy, which goes hand in hand with e-commerce-based drug distribution. Advances in digital health and telemedicine further facilitate this trend by allowing remote consultations and prescriptions. Additionally, the COVID-19 pandemic accelerated the transition to digital platforms, making online pharmacies a norm for managing chronic and seasonal conditions. Regulatory enhancements and increasing confidence in licensed online pharmacy websites are likely to further support this segment's growth in the forecast years.
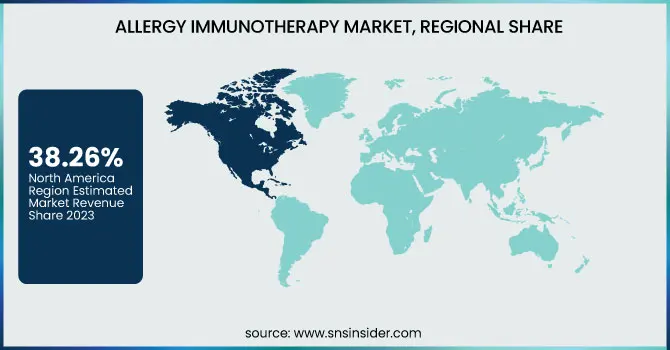
Regional Insights
North America dominated the allergy immunotherapy market with a 38.26% market share in 2023 based on several significant reasons, such as the prevalence of allergic diseases like allergic rhinitis and asthma, well-developed healthcare infrastructure, and favorable regulatory support for immunotherapy-driven treatment solutions. The region is also supported by prominent pharma players actively investing in R&D and commercialization of innovative immunotherapy solutions. Additionally, growing patient awareness, positive reimbursement policies, and increased use of both sublingual and subcutaneous immunotherapy techniques further support the market's growth. The U.S., specifically, is at the center, fueled by efforts to increase access to allergy diagnostics and treatment in clinical and outpatient settings.
The Asia Pacific is anticipated to experience the fastest growth with 9.96% CAGR over the forecast period in the allergy immunotherapy market as a result of the increasing rate of allergic diseases owing to urbanization, pollution, and lifestyle changes. China, India, and Japan are experiencing increased demand for allergy diagnosis and treatment. The region's enhanced healthcare accessibility, enhanced public health consciousness, and rising investments by global and domestic pharma players in allergy treatment infrastructure are the prime drivers. Also, the increase in clinical trials, regulatory clearances for novel therapies, and rising healthcare expenditure in emerging economies are expected to drive the high growth path in the forecast years.
Get Customized Report as per Your Business Requirement - Enquiry Now
Allergy Immunotherapy Market Key Players
-
ALK-Abelló A/S (GRAZAX, ACARIZAX)
-
Stallergenes Greer plc (Oralair, Alustal)
-
Allergy Therapeutics plc (Pollinex Quattro, Venomil)
-
HAL Allergy B.V. (HAL Allergen Extracts, Purethal)
-
LETI Pharma (Oraltek, Prolinair)
-
Biomay AG (BM32, Allergy Vaccine Platform)
-
Torii Pharmaceutical Co., Ltd. (Cedartolen, House Dust Mite SLIT Tablet)
-
Jubilant HollisterStier LLC (HollisterStier Allergen Extracts, Skin Test Antigens)
-
Circassia Group plc (Cat-SPIRE, House Dust Mite-SPIRE)
-
DBV Technologies (Viaskin Peanut, Viaskin Milk)
-
Aimmune Therapeutics (Palforzia, AIMab)
-
Regeneron Pharmaceuticals, Inc. (Dupixent, REGN1908- 1909)
-
Sanofi S.A. (Dupixent, Phadia ImmunoCAP)
-
Allergy Laboratories, Inc. (Allergy Extracts, Allergen Immunotherapy Vials)
-
Creative Diagnostics (Allergen Panel ELISA Kits, Allergen-Specific Antibodies)
-
Biotech Laboratories USA (Solu-Stat, AllerStat)
-
Merck KGaA (Allergovit, Alutard SQ)
-
Inmunotek S.L. (Oralvac, Alxoid)
-
Zydus Lifesciences Ltd. (Zyfex, Zycal)
-
Cipla Ltd. (Allerkid, Asthalin Inhaler)
Suppliers (These suppliers commonly provide essential raw materials, biological reagents, excipients, and analytical testing services critical for the development, quality control, and large-scale manufacturing of allergy immunotherapy drugs and biologics.) in the Allergy Immunotherapy Market
-
Thermo Fisher Scientific
-
Lonza Group AG
-
Merck KGaA (MilliporeSigma)
-
Catalent Inc.
-
Charles River Laboratories
-
WuXi AppTec
-
Eurofins Scientific
-
Fujifilm Diosynth Biotechnologies
-
SGS SA
-
Bio-Rad Laboratories, Inc.
Recent Developments in the Allergy Immunotherapy Market
-
June 2024 – ALK revealed that its Board of Directors has endorsed a new corporate strategy, Allergy+, and its financial aspirations until 2028. The Allergy+ strategy aims to affirm ALK's leadership in the allergy immunotherapy market. The strategy also declares the company's commitment to having a strong footprint in food allergy and anaphylaxis therapy, as well as driving innovation to treat related allergic diseases that still pose critical unmet medical needs.
-
June 2023 – Allergy Therapeutics, a global full-integrated specialty pharmaceutical group committed to allergy immunotherapy, reaffirmed its presence at the European Academy of Allergy and Clinical Immunology (EAACI) Hybrid Congress from June 9–11, 2023, in Hamburg, Germany. The group will highlight its major scientific findings from its research pipeline throughout the congress.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 1.77 Billion |
| Market Size by 2032 | US$ 4.01 Billion |
| CAGR | CAGR of 9.48 % From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Treatment Type (Subcutaneous Immunotherapy, Sublingual Immunotherapy) • By Type (Tablets, Drops) • By Allergy Type (Allergic Rhinitis, Allergic Asthma, Others) • By Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | ALK-Abelló A/S, Stallergenes Greer plc, Allergy Therapeutics plc, HAL Allergy B.V., LETI Pharma, Biomay AG, Torii Pharmaceutical Co., Ltd., Jubilant HollisterStier LLC, Circassia Group plc, DBV Technologies, Aimmune Therapeutics, Regeneron Pharmaceuticals, Inc., Sanofi S.A., Allergy Laboratories, Inc., Creative Diagnostics, Biotech Laboratories USA, Merck KGaA, Inmunotek S.L., Zydus Lifesciences Ltd., Cipla Ltd., and other players. |

