Colorectal Cancer Therapeutics Market Key Insights:
Get More Information on Colorectal Cancer Therapeutics Market - Request Sample Report
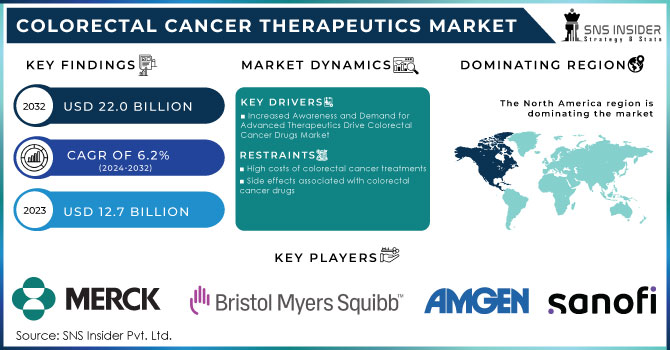
The Colorectal Cancer Therapeutics Market was valued at USD 12.7 billion in 2023 and is expected to reach USD 22.0 billion by 2032 and grow at a CAGR of 6.2% over the forecast period 2024-2032.
Colorectal cancer (CRC) therapeutics market is growing globally due to such various reasons as increased prevalence, advancements in diagnostics and treatments, and increased awareness. According to a study published in the National Library of Medicine in 2021, CRC accounted for an approximate 10% distribution of global cancer cases and 9.4% of the global number of cancer deaths in 2020; the cancer ranked as the second deadliest after lung cancer. The WHO recently reported almost 2 million CRC cases worldwide in 2020. In the U.S., the American Cancer Society projects that 1 in 25 women and 1 in 23 men will develop CRC during their lifetime.
Advances in screening and diagnostics have contributed to a declining CRC mortality while better treatments also contribute to an important factor. In June 2023, the US FDA approved FoundationOne Liquid CDx, which is a genomic profiling test that is designed to identify patients with metastatic CRC harboring BRAF V600E alterations who may benefit from BRAFTOVI and cetuximab therapy. The growing acceptability of advanced therapies such as Avastin, Erbitux, and Stivarga also supports the growth of this market.
Competition and demand are likely to increase, especially due to the patent expirations of biologics, including Avastin, which expired in the United States in 2019 and in Europe in 2022. Biosimilars have entered the oncology space with the introduction of cost-effective alternatives such as Amgen and Allergan's Mvasi. As reported by AJMC, 20 oncology biologics are expected to lose exclusivity by 2023, hence propelling growth in the CRC therapeutics market.
Furthermore, the awareness of CRC coupled with government initiatives for early diagnosis is creating a conducive environment for market expansion. An upward trend will continue to prevail among the advanced treatments and therapies related to the disease in regions like Germany, the U.K., and China, thereby offering opportunities for market players.
Table 1: Recent Clinical Trials in Colorectal Cancer
| Trial Name | Drug Name | Company Name | Phase | Trial Status |
|
APEC |
Atezolizumab |
Roche |
Phase 3 |
Active |
|
DETERMINATION |
Nivolumab |
Bristol-Myers Squibb |
Phase 3 |
Recruiting |
|
CHRONOS |
Regorafenib |
Bayer |
Phase 3 |
Completed |
|
TRIO-012 |
Panitumumab |
Amgen |
Phase 2 |
Active |
|
COLO-REG |
TAS-102 |
Taiho Pharmaceutical |
Phase 3 |
Active |
Table 2: Targeted Therapies for Colorectal Cancer
| Drug Name | Target | Company Name | Trial Name | Phase |
|
Cetuximab |
EGFR |
Merck & Co. |
CRYSTAL |
Phase 3 |
|
Regorafenib |
Multiple kinases |
Bayer |
RESILIENCE |
Phase 3 |
|
Fractalkine |
CX3CR1 |
Axcella Health |
AXS-05 |
Phase 2 |
|
Sunitinib |
VEGFR, PDGFR |
Pfizer |
RESIST |
Phase 2 |
|
Trastuzumab |
HER2 |
Genentech |
HERACLES |
Phase 3 |
Market Dynamics
Drivers
-
Increased Awareness and Demand for Advanced Therapeutics Drive Colorectal Cancer Drugs Market
Increasing awareness of the disease and growing demand for high-tech effective treatment options are driving the global colorectal cancer drugs market. Colorectal cancer is an expensive cancer in terms of mortality. Increasing demand for novel therapeutics that may provide better treatment outcomes among patients diagnosed with colorectal cancer has increased further. A multi-agent approach to treatment is mostly required, involving a combination of drugs, as innovations in drug development continue. The various crucial market players launched several new drugs to cater to the different needs of patients suffering from colorectal cancer. For instance, with the introduction of the Young-Onset Colorectal Cancer Center by Dana-Farber in April 2019 as an improvement measure for patient care, the market is further expanded.
Despite the development of different drugs or treatments, the incidence of colorectal cancer has been increasing steadily and thus constitutes an unmet need in the market because of the requirement for advanced therapies. Although inexpensive generic chemotherapy drugs are available, the disease is complex and severe, so continuous innovation in therapeutics is required. This demand in favor of more effective drugs is a key driver in the global market for colorectal cancer medications.
Restraints
-
High costs of colorectal cancer treatments
-
Side effects associated with colorectal cancer drugs
Market Segmentation Analysis
By Drug Class
Based on drug class, the colorectal cancer drugs market is diversified into chemotherapy, immunotherapy, and others. Immunotherapy accounted for 83.0% of total revenue in 2023 and is expected to grow at the highest CAGR of 5.3% during the forecast period. Immunotherapies are increasingly preferred because they have fewer adverse effects compared with chemotherapy. Such factors as chemotherapy ineffectiveness, side effects of toxicity, and even resistance development are driving immunotherapy to new heights.
Regional Analysis
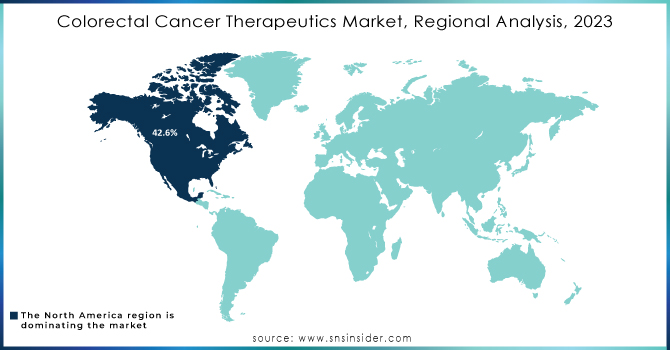
In 2023, North America accounted for the highest revenue share of 42.6% in colorectal cancer drugs. The region's market growth is to remain stable, due to a high incidence of CRC, an increase in treatment rates, and elevated drug prices compared with other regions. Lifestyle factors, such as increasing alcohol consumption, also add to the trend. The International Agency for Research on Cancer (IARC) estimated that about 60% of adults consumed alcohol in 2021, a risk factor that is linked to higher CRC incidence.
Asia Pacific is set to expand with the highest CAGR of 6.7% over the forecast period. This growth is attributed to the rising geriatric population and the growing involvement of public and private organizations in awareness programs about colorectal cancer. For example, a study published by the National Library of Medicine in April 2022 reported various pilot population-based CRC screening programs implemented in different administrative divisions of China, which would help in the early detection and management of the disease.
Need Any Customization Research On Colorectal Cancer Therapeutic Market - Inquiry Now
Key Players
Key Drug Manufacturers for The Colorectal Cancer Therapeutic Market
-
F. Hoffmann-La Roche Ltd.
-
Amgen, Inc.
-
Bayer AG
-
Sanofi
-
Genentech, Inc.
-
Pfizer Inc.
-
Teva Pharmaceutical Industries Ltd.
-
Taiho Pharmaceutical (Otsuka Pharmaceutical Co., Ltd.)
-
Regeneron Pharmaceuticals, Inc.
-
Ipsen Biopharmaceuticals, Inc.
Key Drug Adjuvant Manufacturers for The Colorectal Cancer Therapeutic Market
-
Bristol-Myers Squibb
-
Merck & Co.
-
Amgen
-
Genentech (Roche)
-
Bayer
-
Taiho Pharmaceutical
-
Eli Lilly
-
Pfizer
-
Servier Pharmaceuticals
-
Johnson & Johnson
Key API Manufacturers for The Colorectal Cancer Therapeutic Market
-
Teva Pharmaceutical Industries Ltd.
-
Novartis AG
-
Bristol-Myers Squibb
-
F. Hoffmann-La Roche AG
-
Mylan N.V. (now part of Viatris)
-
Aurobindo Pharma
-
Zydus Cadila
-
Sun Pharmaceutical Industries Ltd.
-
Hikma Pharmaceuticals PLC
-
Apotex Inc.
-
Glenmark Pharmaceuticals
-
Sandoz (a Novartis division)
-
Eisai Co., Ltd.
-
Hetero Labs Limited
-
Cambrex Corporation
Recent Developments
-
April 2024: Taiho Pharmaceutical Co., Ltd. announced promising results from a Phase III clinical trial of its drug LONSURF (trifluridine/tipiracil) for patients with metastatic colorectal cancer, showing improved overall survival rates compared to standard therapies.
-
March 2024: Roche reported advancements in its clinical studies evaluating the combination of Cotellic (cobimetinib) with immuno-oncologic agents, revealing encouraging outcomes in patients with advanced colorectal cancer.
-
February 2024: Regeneron Pharmaceuticals, Inc. initiated a new trial focusing on the efficacy of its PD-1 inhibitor, Libtayo (cemiplimab), in combination with chemotherapy for the treatment of patients with microsatellite instability-high (MSI-H) colorectal cancer.
-
January 2024: Akeda entered into a partnership with Hutchmed, acquiring commercial rights for the colorectal cancer drug fruquintinib outside China, aiming to expand its availability in international markets.
-
December 2023: Bristol-Myers Squibb received FDA approval for a new indication of Opdivo (nivolumab) as a treatment for patients with previously treated metastatic colorectal cancer with specific biomarkers.
-
November 2023: Amgen announced positive Phase II trial results for its investigational drug, which targets specific genetic mutations associated with colorectal cancer, paving the way for future studies and potential market entry.
-
In January 2023, Akeda, a company based in Japan, partnered with Hutchmed, located in Hong Kong, to acquire commercial rights for the colorectal cancer drug fruquintinib outside of China. Additionally, Roche is assessing the combination of its targeted therapy, Cotellic, with immuno-oncologic agents for CRC treatment. These developments are expected to stimulate innovation and drive growth in the market.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 12.7 Billion |
| Market Size by 2032 | US$ 22.0 billion |
| CAGR | CAGR of 6.2% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Drug Class (Immunotherapy, Chemotherapy, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Merck & Co., Inc., F. Hoffmann-La Roche Ltd., Bristol-Myers Squibb Company, Amgen, Inc., Bayer AG, Sanofi, Genentech, Inc., Eli Lilly and Company, Pfizer Inc., Teva Pharmaceutical Industries Ltd., Taiho Pharmaceutical (Otsuka Pharmaceutical Co., Ltd.), Regeneron Pharmaceuticals, Inc., Novartis AG, and others. |
| Key Drivers | • Increased Awareness and Demand for Advanced Therapeutics Drive Colorectal Cancer Drugs Market |
| Restraints | • High costs of colorectal cancer treatments • Side effects associated with colorectal cancer drugs |

