Risk-based Monitoring Software Market Report Scope & Overview:
The Risk-based Monitoring Software Market Size was valued at USD 359.32 Million in 2023, and is expected to reach USD 1004.32 Million by 2031, and grow at a CAGR of 13.71% over the forecast period 2024-2031.
Risk-based monitoring software is a type of clinical trial monitoring software that complies with regulatory criteria. It does, nevertheless, move away from patient data source data verification (SDV). It uses numerous platforms, tools, and dashboards to identify signals that are used to solve issues connected to clinical trial safety, data integrity, and compliance. This risk-based monitoring program aids in the emphasis on trials without putting high-value tasks at risk. The growing number of clinical trials, as well as the increase in government funding and grants for clinical trials, all contribute to the growth of the RBM software market. At the time of speculation, however, the high cost of implementing RBM programs is expected to slow market growth.

Get more information on Risk-based Monitoring Software Market - Request Sample Report
MARKET DYNAMICS
DRIVERS
-
RBM systems are cost- and time-effective.
-
Clinical studies are on the rise.
-
Grants and financing from the government to support clinical trials are being increased.
RESTRAINTS
-
Implementation expenses are high.
OPPORTUNITIES
-
Clinical trial processes are increasingly being outsourced to contract research organisations (CROs).
CHALLENGES
-
Scarcity of qualified personnel to operate RBM solutions
IMPACT OF COVID-19
Medical supplies are in high demand in order to care for the sick populace. Among the most commonly utilised medical equipment in primary clinical care are atomizers, life-support machines, oxygen generators, and monitors. Furthermore, COVID-19 has resulted in a significant increase in demand for medical goods such as masks, gloves, and eye protection. As the number of COVID-19 cases increases around the world, the demand for medical supplies from both healthcare professionals and the general public grows.
By Type
The enterprise and site RBM software markets are divided into two categories. In comparison to the type of site, the enterprise is the largest segment. This software allows academics and professionals to obtain clinical test results from a single location, resulting in high demand from end-users.
By Component
RBM software is divided into two categories: software and services. The RBM software market is likely to be dominated by the software segment. The expanding R&D expenditure in the life science and clinical research industries, as well as an increasing number of clinical trials and a growing client base, can be attributable to this segment's high proportion.
By Delivery Mode
The RBM software market is divided into two categories: Business Licensed (On-premise) and Cloud-based (SaaS). The web-based segment (Wanted) is expected to have a strong presence in the RBM software market. The benefits of web-based software, such as easy access, improved productivity, time efficiency, and cost effectiveness, make up the bulk of this category.
By End-User
Pharmaceutical and biopharmaceutical firms, CROs, medical equipment companies, and other end users are building the RBM software industry. The pharmaceutical and biopharmaceutical industry segment is predicting that it will dominate the RBM software market. An important factor driving the growth of this consumer market is the use of costly R&D for pharmaceutical and biopharmaceutical businesses.
KEY MARKET SEGMENTS:
By Type
-
Site RBM Software
-
Enterprise RBM Software
By Component
-
Services
-
Software
By Delivery Mode
-
Licensed Enterprise (On-premise)
-
Cloud-based (SaaS)
By End-User
-
Pharmaceutical & Biopharmaceutical Companies
-
Medical Device Companies
-
CROs
REGIONAL ANALYSIS
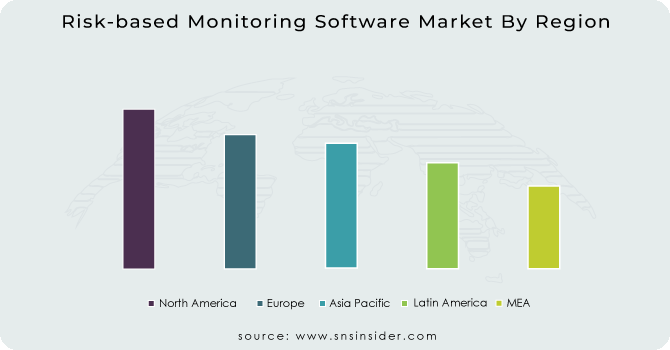
RBM software market in North America is estimated to account for the highest share of the global market. At the time of speculation, the Asia Pacific market was predicted to grow at a rapid pace. This increase may be due to increased government funding for clinical trials, stronger control guidelines than developed countries, greater patient base, lower operating costs for clinical trials, a shortage of volunteers in Europe and North America, and a growing number. of pharmaceutical companies and contract research organizations (CROs) in the region.
Need any customization research on Risk-based Monitoring Software Market - Enquiry Now
REGIONAL COVERAGE:
-
North America
-
USA
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
The Netherlands
-
Rest of Europe
-
-
Asia-Pacific
-
Japan
-
south Korea
-
China
-
India
-
Australia
-
Rest of Asia-Pacific
-
-
The Middle East & Africa
-
Israel
-
UAE
-
South Africa
-
Rest of Middle East & Africa
-
-
Latin America
-
Brazil
-
Argentina
-
Rest of Latin America
-
KEY PLAYERS:
Some of the major key players are as follows: ArisGlobal, Anju Software, Bioclinica, DATATRAK, Forte Research Systems, MedNet Solutions, IBM Corporation, Medidata Solutions, Oracle, Parexe and Other Players.
Bioclinica-Company Financial Analysis
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 359.32 Million |
| Market Size by 2031 | US$ 1004.32 Million |
| CAGR | CAGR of 13.71% From 2024 to 2031 |
| Base Year | 2023 |
| Forecast Period | 2024-2031 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Type (Site RBM Software, Enterprise RBM Software) • By Component (Services, Software) • By Delivery Mode (Licensed Enterprise (On-premise), Cloud-based (SaaS)) • By End-User (Pharmaceutical & Biopharmaceutical Companies, Medical Device Companies, CROs) |
| Regional Analysis/Coverage | North America (USA, Canada, Mexico), Europe (Germany, UK, France, Italy, Spain, Netherlands, Rest of Europe), Asia-Pacific (Japan, South Korea, China, India, Australia, Rest of Asia-Pacific), The Middle East & Africa (Israel, UAE, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Rest of Latin America) |
| Company Profiles | ArisGlobal, Anju Software, Bioclinica, DATATRAK, Forte Research Systems, MedNet Solutions, IBM Corporation, Medidata Solutions, Oracle, Parexel. |
| DRIVERS | • RBM systems are cost- and time-effective. • Clinical studies are on the rise. • Grants and financing from the government to support clinical trials are being increased. |
| RESTRAINTS | • Implementation expenses are high. |

