Vagus Nerve Stimulation Market Report Scope & Overview:
Get More Information on Vagus Nerve Stimulation Market - Request Sample Report
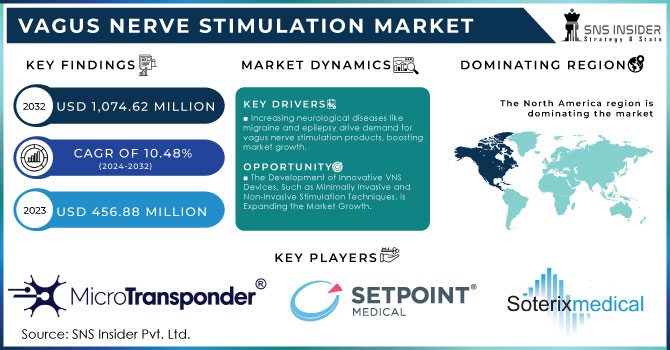
The Vagus Nerve Stimulation Market Size was valued at USD 456.88 Million in 2023, and is expected to reach USD 1,074.62 Million by 2032, and grow at a CAGR of 10.48% over the forecast period 2024-2032.
The market for neurostimulation devices is increasing, which also led to finding a better class that has minimal side effects and thus is optimal therapy. Neurological conditions account for 4.5%-11% of all illnesses as estimated by the WHO, in low or high-income countries alike This figure is greater than that of respiratory diseases, gastrointestinal problems, or certain cancers, and further, his burden will probably experience a rise in the future years. The US has also seen a substantial rise in neurological disorders, and this trend is expected to persist for the next twenty years. Neurological disorders account for a mean of 3200 per 100,000 population and range between approximately as low as only about half this average to twice the (~2–6 in every thousand) prevalence. A common neurological disorder, epilepsy affects millions of people worldwide. This disease is, in most of the populated countries on Earth like India and China. Due to this increasing incidence, the demand for numerous devices and therapies has been on an ever increase like Vagal nerve stimulation (VNS). The therapeutic use of vagal nerve stimulation is expanding, according to Jacobs. It is used in the treatment of neurological diseases such as epilepsy, depression, and other clinical applications. As such, VNS is expected to be part of the treatment for neurological diseases on a global scale.
MARKET DYNAMICS:
KEY DRIVERS:
-
Increasing Incidences of Neurological Diseases Such as Migraine and Epilepsy Leading to Demand for Vagus Nerve Stimulation Products Boost Market Growth.
-
Growing Acceptance of Neuromodulation Therapies is Responsible for the Growth of the Vagus Nerve Stimulation Products Market.
RESTRAINTS:
-
Regulatory Frameworks Often Face Significant Challenges, Such as Bureaucratic Delays, Complex Approval Processes, And Resistance from Industry Stakeholders Impeding the Growth of the Market.
-
Alternative Therapies, Such as Herbal Remedies and Non-Conventional Treatments, Can Compete with Established Medical Treatments.
OPPORTUNITY:
-
The Development of Innovative VNS Devices, Such as Minimally Invasive and Non-Invasive Stimulation Techniques, is Expanding the Market Growth.
-
The Establishment of Favorable Reimbursement Policies in Various Countries is Providing Lucrative Growth Opportunities for The Market
KEY MARKET SEGMENTATION:
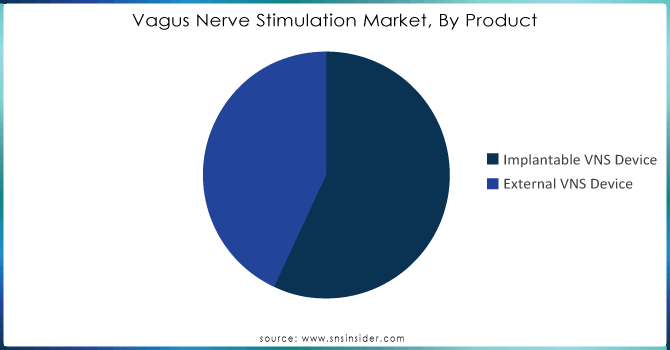
By Product
In 2023, the implantable VNS device segment led in terms of revenue contribution with over a 60% share of this market due growing number of headache, depression, and epilepsy patients across the globe The VNS devices implanted are designed specifically to target the pain site and work by modulating nerve activity through sending an electric stimulation in a particular nerve making it a promising treatment method for this disease. Vagus Nerve Stimulation Market - Based on the product, the vagus nerve stimulation market is divided into implantable and external VNS devices. For people who have not responded to anti-seizure drugs and other intensive depression treatments, such as antidepressant medications, psychotherapy, or electroconvulsive therapy (ECT), an implantable VNS device can be a lifesaver. The launch of novel VNS devices by leading market participants further supports the widespread acceptance and demand for these devices in global markets. For example, MicroTransponder received FDA approval in August 2021 for the Vivistim Paired VNS System which is indicated to treat moderate-to-severe upper extremity motor deficits resulting from chronic ischemic stroke.
Nevertheless, the percentage share of external VNS devices is likely to soar at a CAGR Of 12% between 2024 To 2032. It is mainly due to the growth of patients suffering from chronic diseases and increasing preference for non-invasive treatment as well as cost-effectiveness. Developing new devices, patent pushing towards non-invasive devices changing the patient preference for advanced testing techniques over conventional surgical methods will increase the demand. Adverse events related to implantable VNS devices can span from surgical procedures itself electrical stimulation. As a consequence, non-invasive VNS (nVNS) devices were designed to eliminate the disadvantages associated with implants. Some examples of the non-invasive vagus nerve stimulators that are commercially available include gammaCore Sapphire by electroCore, and Parasym device by Parasym Ltd.
Need any customization research on Vagus Nerve Stimulation Markett - Enquiry Now
By Application
The epilepsy segment dominated the market in 2023, contributing a revenue share of 59% due to rising regulatory approvals and global prevalence rate for epilepsy violence around world-wide for instance, according to the WHO epilepsy is diagnosed in high-income countries with 49 out of 100.000 inhabitants per year and by comparison in low- middle-income countries there are register about 139 people. Additionally, about 100-120 thousand children with epilepsy are hospitalized annually in the United States (US) with associated conditions. This is anticipated to skyrocket the growth of the market. Further, continuous rise in the awareness levels concerning epilepsy treatment together with strategic partnerships are projected to bolster market growth. This has created awareness among the population leading to the higher need for proper diagnoses and treatment in a significant percentage through epilepsy networks. For example, international inauguration; The Epilepsy Association of Nova Scotia joined forces with the Anita Kaufmann Foundation to announce that March 26 was now” Purple Day” internationally and a day for epilepsy specifically.
Medications that are used to treat depression, can be selective serotonin reuptake inhibitors or tricyclic antidepressants. These medications change your chemistry, and have different side effects in different people We all know how the proportion of stress and depression is scaling up on a usual basis throughout the globe. More than 2.7 million American adults carry the weight of treatment-resistant depression, as defined by the National Institute of Mental Health (NIHM). Moreover, According to WHO, more than 300 million adults are facing depression around the world and one-third of patients with major depressive disorder (MDD) have failed treatment-resistant depression (TRD).
By Biomaterial
The metallic biomaterials segment led the market with a 50% revenue share in 2023, owing to the increased R&D for these therapies’ advancements along with the growth of technologies under this application space. The segment will also hold its ground through 2032. Metallic biomaterials: These are commonly used as medical devices and implantable components that give the material its thermal conductivity & mechanical properties. Metals such as 316L stainless steel, titanium-based alloys, gold tantalum, and silver are chosen for the encapsulation of electrical implantable devices due to their capacity to reduce inflammation affecting their conductivity. The polymeric segment is projected to expand at the fastest CAGR of 11%. Depression and stress are on the rise across multiple countries besides ours. Polymers, (such as electro-spun matrices and neural conduits/scaffolds) are being developed to serve as support structures that can regenerate damaged neural tissues.
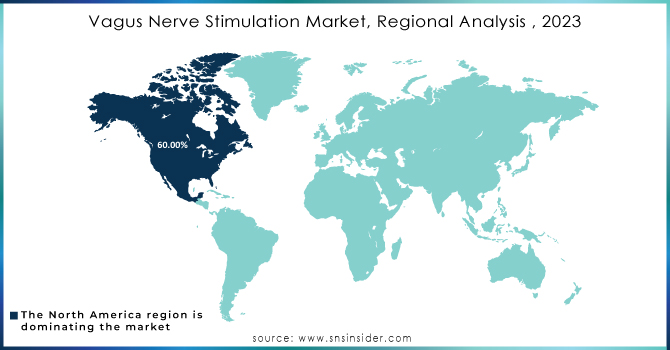
REGIONAL ANALYSIS:
Due to the rise in government funding and initiatives for increasing awareness of epilepsy, stubborn depression, etc., North America accounted for approximately 60% share by revenue based on geography owning the largest shares from the Vagus Nerve Stimulation market. Moreover, the high growth in technological advancements, availability of key manufacturing and R&D hubs in this region with a rise of governmental funding & initiatives are a few drivers that fuel the overall growth of the market. According to CMS - the Centers for Medicare & Medicaid Services, health spending in the U.S. is expected to grow at 5.5% each year from 2018 through its forecast period until ten years later (2027) and reach USD 6 trillion by that final target milestone reached the end of this forthcoming decade (Appendix). Also, high income in developed economies and several qualified professionals are some of the strong factors responsible for North America dominating the market.
The Asia Pacific region is projected to grow at a faster rate. This can be ascribed to the increasing incidence of neurodegenerative diseases in this region and the increased need for innovative solutions that are capable of delivering long-term results. Increasing awareness about treatment options available for neurological diseases and the development of clinical trial infrastructures in emerging APAC countries are expected to register the fastest growth over the forecast period. Further, increasing the number of developing countries like Japan, China, and India would also contribute to positive market growth in high-growth regions. Likewise, the establishment of organizations like the Asia-Pacific Centre for Neuromodulation (APCN) to focus on studies related to VNS benefits is also anticipated to create a high influence on noting growth rates in the region.
KEY PLAYERS:
The key market players include SetPoint Medical Corporation, MicroTransponder Inc., Soterix Medical Inc., Thermo Fisher Scientific, Parasym Ltd., ElectroCore, Inc., Neuropix Company Ltd., TVNS Health GmbH, Beijing PINS Medical Co., Ltd., LivaNova PLC. and other players.
RECENT DEVELOPMENTS
-
In December 2020 ElectroCore, Inc., an established bioelectronic company announced the launch of a new consumer wellness line called Truvaga for patients in the United States.
-
In 2023 SenTiva DUO, is a VNS Therapy solution that is compatible with the implantable pulse generator (IPG) with dual pin header option market-leading medical technology and innovation business LivaNova PLC.
| Report Attributes | Details |
|
Market Size in 2023 |
US$ 456.88 Million |
|
Market Size by 2032 |
US$ 1,074.62 Million |
|
CAGR |
CAGR of 10.48% From 2024 to 2032 |
|
Base Year |
2023 |
|
Forecast Period |
2024-2032 |
|
Historical Data |
2020-2022 |
|
Report Scope & Coverage |
Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
|
Key Segments |
•By Product (Implantable VNS Device, External VNS Device) |
|
Regional Analysis/Coverage |
North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
|
Company Profiles |
Thermo Fisher Scientific, MicroTransponder Inc., Neuropix Company Ltd., Soterix Medical Inc., SetPoint Medical Corporation, Parasym Ltd., ElectroCore, Inc., TVNS Health GmbH, Beijing PINS Medical Co., Ltd., LivaNova PLC. and other players |
|
Key Drivers |
•Increasing Incidences of Neurological Diseases Such as Migraine and Epilepsy Leading to Demand for Vagus Nerve Stimulation Products Boost Market Growth. |
|
RESTRAINTS |
•Regulatory Frameworks Often Face Significant Challenges, Such as Bureaucratic Delays, Complex Approval Processes, And Resistance from Industry Stakeholders Impeding the Growth of the Market. |

