Arbovirus Testing Market Report Scope & Overview:
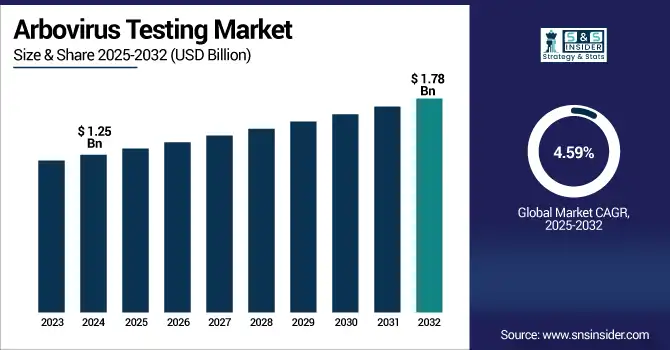
The arbovirus testing market size was valued at USD 1.25 billion in 2024 and is expected to reach USD 1.78 billion by 2032, growing at a CAGR of 4.59% over the forecast period of 2025-2032.
To Get more information on Arbovirus testing market - Request Free Sample Report
Strong government initiatives will drive the demand for the global arbovirus testing market. Surveillance, early detection, and outbreak control are being strengthened by public health authorities, driving demand for reliable diagnostics. It infuses money into research institutions and diagnostic companies so that tests are developed and available. Quality standards are assured by a regulatory system, thus enabling trustworthy diagnostics. Global partnerships, including WHO’s Global Arbovirus Initiative, support acceleration and out-of-the-box thinking. Information drives and upgrading laboratory facilities also increase testing capacity. These initiatives cumulatively increase the uptake of ELISA, RT-PCR, and point-of-care tests; thus, the support of the government is a significant market driver across the globe.
For instance, in October 2024, the CDC boosted arbovirus surveillance funding by USD 90 million, expanding RT-PCR testing in high-risk U.S. states including Florida, Texas, and California.
The U.S. Arbovirus Testing Market was valued at USD 320.84 million in 2024 and is expected to reach USD 453.03 million by 2032, growing at a CAGR of 4.44% over 2025-2032.
The U.S. is dominating the arbovirus testing market as it has a strong integration of Telemedicine to facilitate remote consultation, early symptoms assessment, and ordering of tests, more so in the underserved areas. Virtual care facilitates outbreak surveillance decreases pressures on healthcare resources, and expands the reach of infectious diseases expertise. Improved patient tracking and digital adherence also support disease control. Furthermore, accommodative U.S. federal regulations and expanded reimbursements on telehealth have driven the adoption of remote diagnostic services, thereby enhancing access to RT-PCR and serological arbovirus testing solutions across the nation.
For instance, in April 2025, IN 2024, Telemedicine-based arbovirus consultations in the U.S. rose 48%, surpassing 1.2 million cases in 2024, driven by Medicare support and CDC-led programs.
Market Dynamics:
Drivers:
-
Rising Global Incidence of Dengue and Zika, Driving the Arbovirus Testing Market Growth
The increasing prevalence of dengue and Zika globally is one of the leading factors that is driving the arbovirus testing market growth. Indeed, travel, climate change, and urbanization are expanding the ranges of the mosquito vectors, leading to increased exposure of populations to infection. With the surge in outbreaks, absence of antiviral drugs, and enormous socioeconomic burden, governments are focusing on early detection and infrastructure for diagnosis, which is expected to create a strong demand for arbovirus testing solutions globally.
For instance, in May 2025, WHO reported 6.8 million global dengue cases in early 2025, a 24% rise from 2024, significantly boosting global demand for arbovirus testing solutions.
Restraints:
-
Supply Chain Disruptions, Restraining the Arbovirus Testing Market Growth
Lack of availability of the diagnosis system is a significant factor restraining the arbovirus testing market share. Poor infrastructure, cost, and reliance on central laboratories are constraints for testing in high-burden, low-resource regions. Limited awareness, diagnostic inaccuracies, and inadequate supply also restrict adoption. These impediments slow the detection of outbreaks and limit investment, which, in turn, limits the market opportunity in the regions where arbovirus testing is greatly needed.
For instance, in January 2025, PAHO reported over 40% underreporting of arboviral cases in rural Central America and the Caribbean due to poor lab access and limited trained personnel.
Segmentation Analysis:
By Type
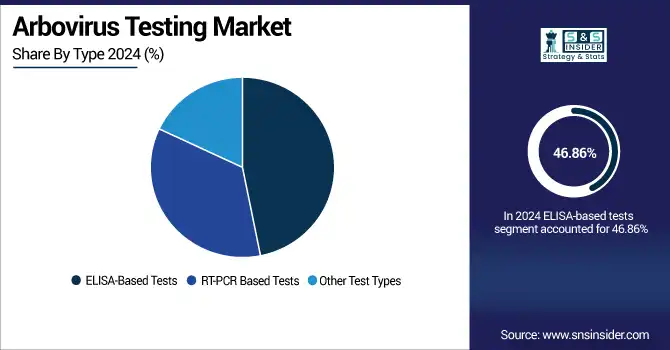
ELISA-based tests were the dominant segment in the arbovirus testing market analysis, with a market share of 46.86% in 2024, as they are inexpensive, scalable, and feasible in large screening programs, in particular in low- and middle-income countries. Their utility for surveillance, outbreak monitoring, and seroprevalence surveys is fuelling mass incorporation into public health systems, with substantial contributions to the total arbovirus testing market share globally.
RT-PCR-based tests are emerging as the fastest-growing segment in the arbovirus testing market trend, with a CAGR of 5.05%, owing to their high sensitivity, precision, and capability of early-infection screening. Their rapid uptake is driven by widespread outbreaks and the need for confirmatory diagnostics, and the growth of lab infrastructure in developing countries. These are substantial factors that are augmenting the global arbovirus testing market growth.
By End-user
In 2024, the Diagnostic Laboratories segment controlled the global arbovirus testing market share, driven by its excellent infrastructure, large-scale testing capacity, and importance for national surveillance and outbreak response. The ability to conduct FR-ELISA and RT-PCR testing with accurate outputs on a large scale has positioned them at the core of public health offering, which in turn, supports expanding the global market of arbovirus testing.
The Research Centers are the fastest-growing segment in the arbovirus testing industry, owing to the rise in R&D spending, new virus mutants, and the demand for new diagnostics, including CRISPR and isothermal tests. Their attention to creating fast, accurate, and field-ready tests enables early detection and global preparedness, and they are a major factor in fueling the global arbovirus testing market growth.
Regional Analysis:
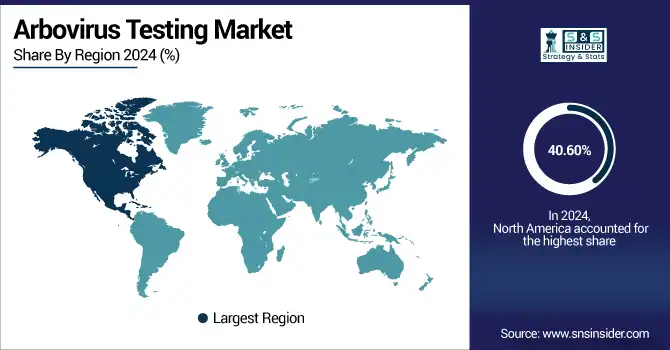
In 2024, the North American region dominated the arbovirus testing industry and accounted for 40.60% of the overall revenue share, owing to awareness of public and the early disease reporting systems in the region. In the U.S. and Canada, there is stronger education, travel advisories, and proactive public health alerts, so it is more likely that the patients and health care providers will pursue earlier testing. This leads to increased testing volume, more timely diagnosis, and rapid response in case of an outbreak, thus driving the overall demand for routine and emergency diagnostic test solutions. It also relies on technology preparedness, which will substantially increase North America's arbovirus testing market share due to the awareness-centric mode of its system.
The arbovirus testing market in Europe is growing steadily, owing to the spread of Aedes mosquitoes into southern and central Europe following climate change and warm temperatures. This has caused dengue outbreaks in Italy, France, and Spain. Compounding this are high levels of international travel, early uptake of diagnostics, and government-sponsored surveillance programmes driving testing volumes higher. The area is also spending on research and public health preparedness that will help to develop advanced-testing capabilities and deploy those technologies.
The Asia Pacific region is projected to grow with the fastest CAGR of 5.47% over the forecast period, owing to its high burden of endemic diseases, including dengue, Zika, and chikungunya, particularly in India, Indonesia, Thailand, and the Philippines. Due to recurrent epidemics, increased rate of urbanization, and disease-promoting effects of climate change, there has been an increased demand for early and accurate diagnosis. Governments around the region are pouring resources into public health infrastructure, mobile diagnostic labs, and point-of-care testing, to reach rural and remote areas. Moreover, increasing healthcare awareness, cross-national co-operation, and endorsements from the WHO and ASEAN will promote the adoption of testing. Large population pool and increasing healthcare spending in the region are also contributing to this growth, and thus, APAC is becoming an important market for the global arbovirus testing industry in the upcoming future.
The Middle East & Africa is the least attractive regions in the arbovirus testing market, owing to poor healthcare infrastructure, lack of diagnostic facilities, and low public health expenditure. Several areas do not have RT-PCR or ELISA capability and are dependent on minimum surveillance systems. Under-reporting, low patient awareness of the disease, and logistical difficulties in rural and remote areas hamper testing coverage, which prevents wide market penetration and overall growth in this high-burden but underserved region.
Latin America has a significant share in the global arbovirus testing industry, with a high prevalence of arboviral diseases including dengue, Zika, and chikungunya. Countries including Brazil, Mexico, and Colombia are hit by mass outbreaks and hence have a high need for diagnoses. Governments in the region have rolled out national surveillance programs, stepped up public health budgets, and disbursed millions of ELISA and RT-PCR test kits to fight the spiking infection rates. Urban crowding, the tropical climate, and the widespread infestation of Aedes aegypti mosquitoes are likely to ensure continued transmission and testing pressure.
Get Customized Report as per Your Business Requirement - Enquiry Now
Key Players:
Arbovirus testing companies include Abbott Laboratories, F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., Siemens Healthineers AG, QIAGEN N.V., Cepheid, bioMérieux SA, InBios International, Inc., Meridian Bioscience, Inc., and other players.
Recent Developments:
-
In April 2025, Thermo Fisher launched the TaqPath Tropical Virus Panel, a CE-marked RT-PCR assay for dengue, Zika, and chikungunya, enhancing molecular diagnostics and boosting the arbovirus testing market.
-
In March 2024, QIAGEN partnered with ICMR to develop a portable multiplex RT-PCR panel for dengue, chikungunya, and Japanese encephalitis, supporting outbreak control and expanding the arbovirus testing market.
| Report Attributes | Details |
| Market Size in 2024 | USD 1.25 billion |
| Market Size by 2032 | USD 1.78 billion |
| CAGR | CAGR of 4.59% From 2025 to 2032 |
| Base Year | 2024 |
| Forecast Period | 2025-2032 |
| Historical Data | 2021-2023 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | "• By Type (ELISA-Based Tests, RT-PCR Based Tests, Other Test Types) •By End-user(Diagnostic Laboratories, Hospitals, Research Centers, Other End Use)" |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, Poland, Turkey, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Rest of Latin America) |
| Company Profiles | Abbott Laboratories, F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., Siemens Healthineers AG, QIAGEN N.V., Cepheid, bioMérieux SA, InBios International, Inc., Meridian Bioscience, Inc.,and other players |

