Cell & Gene Therapy Manufacturing Services Market Report Scope & Overview:
Get more information on Cell & Gene Therapy Manufacturing Services Market - Request Sample Report
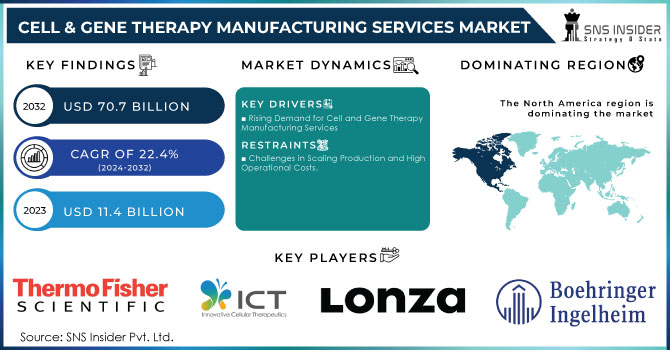
The Cell & Gene Therapy Manufacturing Services Market Size was valued at USD 11.4 billion in 2023 and is expected to reach USD 70.7 billion by 2032 and grow at a CAGR of 22.4% over the forecast period 2024-2032.
Cell & Gene Therapy Manufacturing Services Market: Rapid Growth Driven by Innovative Treatments and Expanding Clinical Trials
Surge in Cell and Gene Therapies Reshaping Biopharmaceutical Landscape
The Cell & Gene Therapy Manufacturing Services Market is witnessing substantial growth, primarily driven by groundbreaking advancements in therapeutic approaches for life-threatening and rare diseases. This surge is largely attributed to the swift evolution of cell and gene therapies, which are revolutionizing the biopharmaceutical sector by enhancing clinical success rates and increasing the number of therapies in development. As of May 2022, 329 cell and gene therapies were undergoing clinical trials, with this number expected to rise significantly due to improved scientific understanding and clinical practices. A key growth driver is a notable investment in gene therapy companies, with approximately USD 2.3 billion invested over the past decade, showcasing a robust commitment from global pharmaceutical and biotechnology firms. The market is also supported by the increasing prevalence of cancer and other target diseases, alongside heightened R&D spending by pharmaceutical companies. For instance, GLOBOCAN 2020 reported a global cancer burden of 19.3 million cases and 10 million deaths in 2020, with projections indicating over 50 million new cancer cases in the next five years. The rise in breast cancer cases further underscores the urgent need for innovative treatment options. Cell and gene therapies, such as immunotherapy, gene transfer, and oncolytic virotherapy, offer promising solutions for cancer treatment. With approximately 1,200 cell and gene therapies in global clinical trials and over 700 in the USA, the demand for manufacturing facilities is surging. However, the current infrastructure is inadequate, necessitating the establishment of numerous additional facilities to meet growing demand and address the high costs associated with viral vector production. Although the COVID-19 pandemic impacted the cell and gene therapy manufacturing sector by disrupting supply chains and introducing capacity limitations, it also accelerated the development of new therapies, such as the FDA-approved BCDA-04 for COVID-19-related acute respiratory distress syndrome. As the pandemic recedes, the market is expected to return to pre-pandemic growth levels. Factors driving market expansion include increasing cancer cases in countries like China, the United States, and Japan, where rising cancer incidence rates, such as colorectal cancer, are expected to drive demand for gene and cell therapies. Additionally, the growing prevalence of orthopedic disorders like osteoporosis, which could benefit from stem cell therapies, supports market growth. The establishment of new cell therapy manufacturing facilities, like OrganaBio’s GMP facility in June 2022, further signifies the sector's growth potential. Despite these positive trends, high operational costs for cell and gene therapy manufacturing, including production, research, development, clinical trials, and commercialization expenses, present a significant challenge. Nevertheless, the combination of increasing disease prevalence, rising R&D investments, and ongoing advancements in manufacturing capabilities is expected to drive substantial growth in the cell and gene therapy manufacturing services market in the coming years.
Market Dynamics
Drivers
-
Rising Demand for Cell and Gene Therapy Manufacturing Services:
The expansion of the cell and gene therapy manufacturing services market is primarily driven by the increasing incidence of chronic and life-threatening conditions, such as cancer, genetic disorders, and cardiovascular diseases, which necessitate advanced treatments. The surge in clinical trials and the growing pipeline of cell and gene therapies have significantly elevated the need for specialized manufacturing capabilities. With over 1,200 ongoing clinical trials, scaling up production to meet future demands is crucial. Additionally, heightened investments from both public and private sectors are accelerating market growth, as governments and biopharma companies invest in advancing manufacturing technologies. Innovative manufacturing methods, such as automation and closed systems, are improving process efficiency and scalability. The rising reliance on contract development and manufacturing organizations (CDMOs) among smaller biotech firms, which lack large-scale production infrastructure, is also driving outsourcing in the sector. Furthermore, the healthcare needs of aging populations and the move towards personalized medicine are major factors fueling demand for cell and gene therapies.
Restraints
-
Challenges in Scaling Production and High Operational Costs:
The significant operational costs associated with cell and gene therapy manufacturing, including production, research, development, clinical trials, and commercialization expenses, present a notable challenge.
Key Segmentation
By Therapy Type
-
Cell Therapy:
In 2023, the cell therapy manufacturing segment dominated the market with a commanding revenue share of 59.9%. This leading position is attributed to the influx of new products entering the market and the substantial number of ongoing clinical trials. Over 360 clinical trials are currently investigating CAR-T cell therapies and other cell-based treatments, underscoring the significant potential of these therapies across various disease indications. Consequently, the demand for advanced therapy manufacturing services is expected to rise substantially. Conversely, the gene therapy segment is poised for remarkable growth, with a significant Compound Annual Growth Rate (CAGR) projected during the forecast period. This growth is driven by the large number of products in clinical trials and the need for improvements in gene therapy production processes. Increasing investments and the clinical success of numerous products are driving gene therapy companies to focus on manufacturing and commercialization. Noteworthy strategic moves, such as Pfizer’s September 2020 collaboration with Vivet Therapeutics to manufacture a candidate for Wilson's disease, illustrate the active efforts of global companies to enhance their presence in the gene therapy manufacturing market.
By Manufacturing Scale
-
Pre-Commercial/R&D Scale:
In 2023, the pre-commercial/R&D scale manufacturing segment captured a substantial revenue share of 72.34%. This dominance is driven by the expanding pipeline of gene and cell therapies and increasing investments, positively impacting segment growth. By 2021, over 2,073 clinical trials for various cell therapies were underway, and over 200 gene therapies were in clinical trials as of September 2021. This robust pipeline is expected to yield up to 40 new products approved for clinical use over the next decade. Meanwhile, the commercial-scale manufacturing segment is projected to experience the highest CAGR during the forecast period. The rapid increase in regulatory approvals for gene and cell therapy products is driving a surge in demand for commercial production. Major industry players, such as Thermo Fisher Scientific and AGC Biologics, are actively pursuing strategic initiatives to meet this growing demand. For example, in February 2022, Thermo Fisher Scientific introduced new Patheon Commercial Packaging Services for cell and gene therapy across the U.S. and Europe, creating a favorable environment for significant growth in the commercial-scale manufacturing segment by 2032.
By Manufacturing Mode
-
Contract Manufacturing:
In 2023, the contract manufacturing mode segment led the market with a significant revenue share of 66.3%. This dominance is driven by the increasing demand for cell and gene therapies and the resultant shortage in manufacturing capacity, creating substantial opportunities for contract manufacturing service providers. Given the non-standardized and rapidly evolving nature of the cell and gene therapy market, outsourcing to specialized contract manufacturers offers a competitive edge through their expertise and experience. As noted in a March 2020 article, approximately 35% of traditional biologics manufacturing processes are outsourced, while over 65% of cell and gene therapy manufacturing is outsourced. This high outsourcing rate is due to many small firms, which are at the forefront of innovation in this field, lacking the capacity, expertise, and resources necessary for commercialization. The ever-expanding clinical pipeline further accelerates growth in this segment. Conversely, the in-house manufacturing segment is expected to exhibit the highest CAGR during the forecast period. This growth is supported by academic institutions with personalized treatment programs and entities with substantial capital investments. Additionally, the advantages of in-house manufacturing over contract manufacturing, such as greater control and integration, are likely to enhance segment growth. Small biotechnology companies developing cell and gene therapies often partner with contract manufacturers due to limited resources and infrastructure, but increasing investments of 30-35% annually in in-house capabilities are set to drive notable growth in this segment in the coming years.
By Workflow
-
Process Development:
In 2023, the process development segment captured a significant revenue share of 16.97%. This prominence is attributed to the growing number of therapies transitioning from clinical trials to regulatory approval, underscoring the necessity for well-characterized and robust production methods for cell therapies. Effective process development is crucial for enhancing the efficiency, quality, and safety of candidate programs. It encompasses all aspects of the production process, including cell characterization, isolation, optimization of culture media, impurity removal, and scale-up. Conversely, the vector development segment is anticipated to experience the fastest CAGR during the forecast period. The rapid expansion of manufacturing services in this area is a key driver of growth. As the market for gene therapies continues to grow, so does the demand for viral vectors. These vectors, including adenoviral, retroviral, AAV, lentiviral, and herpes simplex virus, are essential for treating various disorders, including metabolic, cardiovascular, muscular, infectious, hematologic, and ophthalmologic diseases, as well as cancers. The high utilization and ongoing advancements in viral vector technology are expected to drive significant growth in this segment.
Regional Analysis
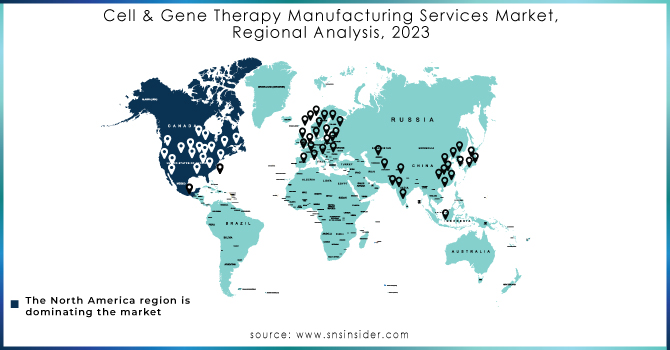
In 2023, North America led the market with the highest revenue share of 47.42%. This dominance is attributed to the region's active involvement in gene and cell therapy research and development and the presence of numerous contract development organizations. Additionally, many domestic companies are expanding their manufacturing capabilities within North America. Meanwhile, the Asia Pacific region is poised for significant cell and gene therapy manufacturing sector growth. It is expected to achieve the fastest growth rate, with a notable CAGR projected from 2024 to 2032. The cell therapy market in Asia is gaining momentum due to accelerated approval pathways, rising healthcare demands, and increasing investments from both private and government sectors.
Need any customization research on Cell & Gene Therapy Manufacturing Services Market - Enquiry Now
Key Players
-
Cellular Therapeutics
-
Lonza
-
Bluebird Bio Inc.
-
Thermo Fisher Scientific
-
Samsung Biologics
-
Boehringer Ingelheim
-
Hitachi Chemical Co., Ltd.
-
Takara Bio Inc.
-
Catalent Inc.
-
Miltenyi Biotec
-
F. Hoffmann-La Roche Ltd
-
Novartis AG
-
Merck KGaA
- Wuxi Advanced Therapies and others.
Recent Developments
-
In June 2022, OrganaBio expanded its GMP cell therapy manufacturing facility in Florida to meet the growing demand for cell and gene therapies.
-
In July 2023, Allogene Therapeutics announced the completion of its new manufacturing facility in Newark, California, enhancing its cell therapy production capabilities.
-
In August 2023, Pfizer acquired a majority stake in a gene therapy company, strengthening its position in the gene therapy manufacturing sector.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 11.4 Billion |
| Market Size by 2032 | US$ 70.7 Billion |
| CAGR | CAGR of 22.4% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Therapy Type [Cell therapy manufacturing (Stem cell therapy, Non stem cell therapy), Gene therapy manufacturing] • By Manufacturing Scale [Pre-commercial/R&D scale manufacturing, Commercial scale manufacturing] • By Manufacturing Mode [Contract manufacturing, In-house manufacturing] • By Workflow [Cell processing, Cell banking, Process development, Fill & finish operations, Analytical and quality testing, Raw material testing, Vector production, Others] |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles |
Miltenyi Biotec, Cellular Therapeutics, Thermo Fisher Scientific, Samsung Biologics, Takara Bio Inc., F. Hoffmann-La Roche Ltd., Hitachi Chemical Co., Ltd., Catalent Inc., Wuxi Advanced Therapies, Lonza, Bluebird Bio Inc., Boehringer Ingelheim, Novartis AG, Merck KGaA and others. |
| DRIVERS | • Rising Demand for Cell and Gene Therapy Manufacturing Services |
| RESTRAINTS | • Challenges in Scaling Production and High Operational Costs |

