Geographic Atrophy (GA) Market Report Scope & Overview:
Get more information on Geographic Atrophy (GA) Market - Request Sample Report
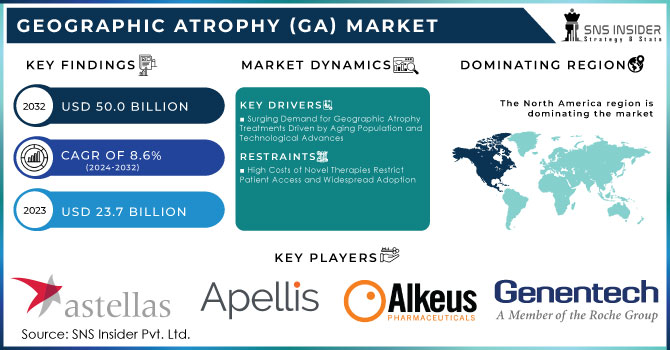
The Geographic Atrophy (GA) Market Size was valued at USD 23.7 Billion in 2023 and is expected to reach USD 50.0 Billion by 2032 and grow at a CAGR of 8.6% over the forecast period 2024-2032.
Robust Growth in the Geographic Atrophy Market Driven by Aging Population and Technological Advances
Heightened Focus on R&D and Novel Therapies Propel Market Expansion
The Geographic Atrophy (GA) market is experiencing robust growth, fueled by several dynamic factors. Geographic atrophy, a severe form of age-related macular degeneration (AMD), poses a significant global health challenge. According to the American Academy of Ophthalmology, over 8 million people worldwide suffer from GA, which constitutes approximately 20% of all AMD cases. The prevalence of GA is anticipated to rise due to the expanding geriatric population, which is more vulnerable to age-related diseases. The increasing age burden in developed nations amplifies the need for effective GA treatments, driving market growth.
A major catalyst for market expansion is the heightened focus on research and development (R&D) by pharmaceutical and biopharmaceutical companies. These entities are investing heavily in innovative therapies to address GA, spurred by the growing incidence of the condition and the urgent need for effective treatment options. Recent advancements in healthcare technology and novel drug development are accelerating market growth. Despite these advancements, the market faces challenges, including limited awareness about GA and disappointing outcomes from some investigational drugs. These challenges have occasionally hindered market progress, yet ongoing R&D and increased health awareness are expected to address these gaps and drive growth.
| Category | Key Drivers/Opportunities | Expected Impact (2024–2032) | Remarks |
|---|---|---|---|
| Aging Population | Increasing global geriatric population | High: Boosting demand for GA therapies | Rising AMD prevalence among elderly |
| FDA Approvals | Approval of novel treatments like IZERVAY | High: Accelerates access to advanced treatments | Improves patient outcomes and treatment reach |
| R&D Investment | Increased focus on research and innovative therapies | Moderate-High: Supports development of new drugs | Leading to breakthrough therapies |
| Gene Therapy Advancements | Innovations in gene and stem cell therapies | High: Potential for disease-modifying solutions | Emerging field with long-term potential |
| Growing Healthcare Awareness | Improved awareness & early diagnosis | Moderate: Enhances early-stage detection | Leads to timely intervention and better management |
| Healthcare Infrastructure | Expansion of healthcare systems in emerging markets | Moderate-High: Expands treatment access globally | Key regions: Asia-Pacific, Latin America |
| Collaborations & Partnerships | Global pharma collaborations on clinical trials | High: Strengthens drug development pipelines | Facilitates faster innovation |
A significant recent development is the FDA's approval of IZERVAY (avacincaptad pegol intravitreal solution) in August 2023. This novel complement C5 inhibitor, developed by Astellas Pharma Inc., has shown promising results in clinical trials. The GATHER1 and GATHER2 Phase 3 trials, assessing the safety and efficacy of monthly 2 mg intravitreal administration of IZERVAY, demonstrated a statistically significant reduction in GA progression. Patients receiving IZERVAY exhibited a slower GA growth rate compared to those receiving sham treatment, marking a milestone in the treatment of this debilitating condition. This approval underscores the market's potential for new and effective therapies, with regulatory bodies playing a crucial role in facilitating development. The market also benefits from rising health awareness and growing healthcare infrastructure globally. As healthcare systems expand and awareness about GA increases, the demand for effective treatments is expected to rise. The growth rate reflects the increased focus on developing novel therapies and the growing incidence of GA due to an aging population. Overall, the geographic atrophy treatment market is poised for substantial growth, driven by increased disease prevalence, advancements in treatment options, and significant R&D investments. The recent FDA approval of IZERVAY highlights the market's potential for new therapies, while regional dynamics and healthcare advancements continue to shape its trajectory.
| Company | Product/Drug | Innovation/Technology | Positive Impact on GA Market |
|---|---|---|---|
| Iveric Bio | Zimura (avacincaptad pegol) | Complement C5 inhibitor targeting GA | FDA approval demonstrates effectiveness in slowing GA progression |
| Apellis Pharmaceuticals | Pegcetacoplan (Syfovre) | Inhibition of complement protein C3 | Approved as a new treatment for slowing GA progression |
| Alkeus Pharmaceuticals | ALK-001 | Vitamin A deuterium-based therapy to protect retinal cells | Innovative approach reduces toxicity, preserving visual function |
| Gyroscope Therapeutics | GT005 | Gene therapy for complement system regulation | Potential long-term impact on preventing GA progression |
| Stealth BioTherapeutics | Elamipretide | Mitochondria-targeted therapy to protect photoreceptors | First-of-its-kind therapy aimed at cellular energy restoration |
| NGM Biopharmaceuticals | NGM621 | Anti-complement C3 monoclonal antibody | Promising results in targeting complement pathways to slow GA |
| Astellas Pharma | IZERVAY (avacincaptad pegol) | Complement C5 inhibitor | Statistically significant reduction in GA progression in clinical trials |
| Ocugen | OCU410 | Modifier gene therapy for GA | Innovative gene therapy addressing underlying causes of GA |
| Annexon Biosciences | ANX007 | Complement C1q inhibitor targeting early stages of GA | First drug to target complement component C1q in GA |
Market Dynamics
Drivers
-
Surging Demand for Geographic Atrophy Treatments Driven by Aging Population and Technological Advances
The geographic atrophy (GA) market is witnessing significant growth due to several key drivers. One primary factor fueling market expansion is the rising prevalence of age-related macular degeneration (AMD), the leading cause of GA. As the global population ages, the incidence of AMD and consequently GA is increasing, creating a higher demand for effective treatments. Advances in medical research and technology are also driving market growth. The development of novel therapies, including gene therapies, stem cell treatments, and targeted drug delivery systems, offers hope for more effective GA management. Increased investment in R&D by pharmaceutical companies and healthcare organizations is accelerating innovation in treatment options.
Furthermore, growing awareness and early diagnosis of GA contribute to market growth. Improved diagnostic tools and increased screening programs help identify GA earlier, allowing for timely intervention and treatment. The expansion of healthcare infrastructure and advanced medical facilities in emerging markets also contribute to market growth. Additionally, rising healthcare expenditure and favorable reimbursement policies in various regions support patient access to innovative treatments. The convergence of these factors creates a robust environment for the geographic atrophy treatment market, driving the development of new therapies and the expansion of existing treatment options.
Restraints
-
High Costs of Novel Therapies Restrict Patient Access and Widespread Adoption
A significant challenge is the high cost associated with novel therapies and advanced treatments, which limits patient access and can be a barrier to widespread adoption. Additionally, the complexity and prolonged development timelines of cutting-edge treatments, such as gene and stem cell therapies, contribute to delays in market availability. Regulatory hurdles and stringent approval processes further complicate the introduction of innovative treatments. A lack of awareness and understanding of GA among the general population and some healthcare providers can lead to underdiagnosis and delayed treatment. These factors collectively constrain the growth potential of the GA treatment market, despite the overall positive outlook.
Key Segmentation
By Age Group
In the Geographic Atrophy (GA) market, segmentation by age group reveals that individuals aged 75 years and older dominate the market. This segment held the largest market share in 2023 with a 54.7% share due to the higher prevalence of geographic atrophy in this age group, driven primarily by age-related factors. According to the Retina Study Group of Portugal, geographic atrophy affects 1.3% of adults aged 75 to 84, with prevalence increasing to approximately 22% by the age of 90. This trend underscores the significant market potential within the older age bracket. The segment of individuals aged 75 and older is not only the largest but also the fastest-growing segment in the GA market, reflecting the growing need for targeted treatments for this demographic. In a notable development, Iveric Bio's investigational drug, avacincaptad pegol (ACP), also known as Zimura, received the Breakthrough Therapy designation from the U.S. Food and Drug Administration (FDA) in November 2021. This novel complement C5 inhibitor is specifically designed for the treatment of geographic atrophy secondary to age-related macular degeneration (AMD), highlighting the market's focus on advanced treatments for this high-prevalence age group.
By Diagnosis
In the Geographic Atrophy (GA) market, segmentation by diagnostic methods reveals that optical coherence tomography angiography (OCT-A) is the dominant and fastest-growing segment. In 2023, OCT-A led the market with 38.6% due to its advanced, non-invasive technology that offers detailed imaging of the retina's and choroid's microvasculature. This capability makes OCT-A essential for diagnosing and understanding various retinal conditions, including geographic atrophy, diabetic retinopathy, and both dry and wet age-related macular degeneration. The technology’s growing importance is reflected in its projected rapid expansion through 2030. OCT-A’s ability to provide high-resolution images and its utility in monitoring disease progression are key factors driving its market dominance. For instance, the AngioVue OCTA system from Optovue, Inc. (US) exemplifies how OCT-A technology is utilized to enhance diagnostic precision and patient care in retinal disorders.
By Therapeutic Agents
In the Geographic Atrophy (GA) market, the segmentation by clinical development stage includes late-stage (Phase III), Phase II, Phase I, and pre-clinical stage & discovery candidates. The late-stage (Phase III) segment was the market leader in 2023 with 34.5% and is anticipated to be the fastest-growing segment from 2024 to 2032. This growth is driven by the pivotal role of Phase III clinical trials, which assess whether new treatments or drug combinations are more effective or comparable to existing therapies. For example, Galderma Laboratories LP (US) is conducting Phase III trials of an anti-inflammatory doxycycline for GA treatment. Additionally, Apellis Pharmaceuticals, Inc. (US) reported promising results from its Phase III trial for APL-2 Therapy in September 2021 and planned to submit a new drug application to the FDA in early 2022. The increasing number of late-stage clinical trials is a significant factor contributing to the expansion of this segment in the GA market.
Regional Analysis
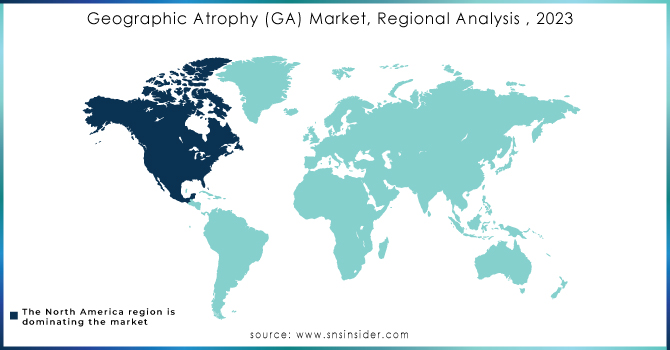
North America, particularly the United States, held the largest share 43.6% of the Geographic Atrophy (GA) market in 2023 and is expected to maintain its dominance through the forecast period of 2024-2032. The region's leadership is attributed to the high prevalence of geographic atrophy, significant investments in healthcare infrastructure, and robust R&D activities. The FDA’s approval of innovative treatments, such as the Phase III trials for APL-2 Therapy by Apellis Pharmaceuticals, further fuels market growth. Additionally, the presence of leading pharmaceutical companies and advanced healthcare systems enhances North America's market position.
The Asia-Pacific region is emerging as a rapidly growing market for geographic atrophy treatments. The region’s growth is fueled by increasing healthcare awareness, expanding healthcare infrastructure, and rising prevalence of age-related diseases. Countries such as China and Japan are leading in market expansion due to their large geriatric populations and significant investments in healthcare technology. The presence of local pharmaceutical companies and increasing collaborations with global firms contribute to the region's rapid market development.
Need any customization research on Geographic Atrophy (GA) Market - Enquiry Now
Key Players
-
Iveric Bio (Zimura (avacincaptad pegol))
-
Alkeus Pharmaceuticals, Inc. (ALK-001)
-
Apellis Pharmaceuticals, Inc. (APL-2)
-
Genentech, Inc. (OpRegen)
-
Stealth BioTherapeutics (Elamipretide)
-
Allegro Ophthalmics, LLC (Risuteganib)
-
Gyroscope Therapeutics Limited (GT005)
-
Regenerative Patch Technologies, LLC (CPCB-RPE1)
-
NGM Biopharmaceuticals Inc. (NGM621)
-
Novartis (PPY988)
-
Perceive Biotherapeutics, Inc. (VOY-101)
-
Annexon, Inc. (ANX007)
-
Aviceda Therapeutics (AVD-104)
-
Ionis Pharmaceuticals, Inc. (IONIS-FB-LRx)
-
Astellas Pharma Inc. (ASP7317), and others.
Recent Developments
-
In April 2024, Ocugen completed dosing for Cohort 2 of the Phase 1/2 Arcellx study on their novel therapy for GA.
-
In July 2024, the National Eye Institute announced a new research initiative to explore gene therapy options for geographic atrophy.
-
In August 2024, Apellis Pharmaceuticals reported successful results from their Phase III clinical trial of APL-2, advancing toward potential FDA approval.
-
In September 2024, Iveric Bio received FDA approval for Zimura, a complement C5 inhibitor, marking a significant milestone in GA treatment.
-
February 2023: Pegcetacoplan (Syfovre) received approval as a new treatment for dry AMD, which can progress to geographic atrophy and cause vision loss. This newly approved medication aims to slow disease progression.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 23.7 Billion |
| Market Size by 2032 | US$ 50.0 billion |
| CAGR | CAGR of 8.6% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Age Group (Above 60 Years, Above 75 Years) • By Diagnosis (Fundus Autofluorescence (FAF), Optical Coherence Tomography Angiography (OCT-A), Multifocal Electroretinography (mfERG)) • By Therapeutic Agents (Late-stage (Phase III), Phase II, Phase I, Pre-clinical stage & Discovery candidates) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Iveric Bio, Alkeus Pharmaceuticals, Inc., Apellis Pharmaceuticals, Inc., Hemera Biosciences LLC, Genentech, Inc., F. Hoffmann-La Roche AG, Stealth BioTherapeutics, Allegro Ophthalmics, LLC, Gensight Biologics SA, Gyroscope Therapeutics Limited, Regenerative Patch Technologies, LLC, NGM Biopharmaceuticals Inc., Novartis, Perceive Biotherapeutics, Inc., Annexon, Inc., Aviceda Therapeutics, Astellas Pharma Inc and others. |
| Key Drivers | • Surging Demand for Geographic Atrophy Treatments Driven by Aging Population and Technological Advances |
| Restraints | • High Costs of Novel Therapies Restrict Patient Access and Widespread Adoption |

