Global Psoriasis Treatment Market Size & Overview:
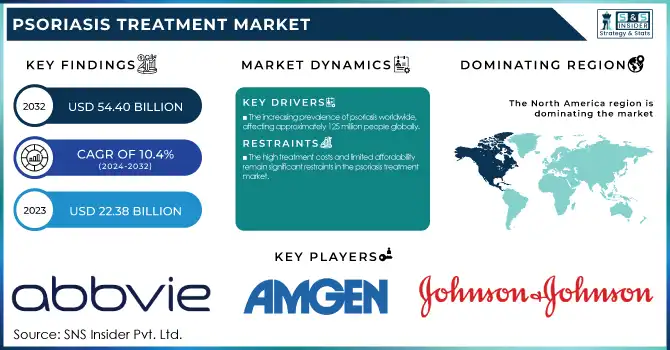
The Psoriasis Treatment Market size was valued at USD 22.38 billion in 2023 and is projected to reach USD 54.40 billion by 2032, growing at a CAGR of 10.4% during the forecast period 2024-2032.
Get More Information on Psoriasis Treatment Market - Request Sample Report
This report shows the increasing prevalence and incidence of psoriasis, as well as distribution of severity and rate of diagnosis, impacting treatment demand. Regional patterns of treatment adoption trends are the subject of this research, highlighting disparities in access to topical treatments, biologics, and systemic therapies. The research also looks at the effect of biologic patent expiration and the increasing presence of biosimilars, which are redefining market rivalry and price dynamics. The report also addresses patient preference and satisfaction trends, indicating a move toward more personalized treatment strategies and better long-term disease control. In addition, it assesses the growing role of digital health and telemedicine in psoriasis management, improving patient activation, remote consultations, and therapy compliance.
Psoriasis Treatment Market Dynamics
Drivers
-
The increasing prevalence of psoriasis worldwide, affecting approximately 125 million people globally.
The increasing adoption of biologic treatments, such as TNF and IL inhibitors, has resulted in improved management of the disease, resulting in increased treatment needs. New advances in drug discovery, such as targeted immunotherapies and JAK inhibitors, have improved treatment options and patient outcomes. Increased awareness drives by organizations like the National Psoriasis Foundation (NPF) and the International Federation of Psoriasis Associations (IFPA) have enhanced early diagnosis and increased patient access to sophisticated treatments. In addition, pharma firms are heavily investing in R&D and clinical trials, and medicines such as Bimekizumab (UCB) and Mirikizumab (Eli Lilly) are yielding positive results. The trend towards personalized medicine and precision therapies is also a major driving force, enhancing efficacy while minimizing side effects. In addition, increasing healthcare coverage and insurance reimbursement policies in developed and emerging markets are making treatment more accessible. The increasing use of teledermatology and electronic health solutions is also making it easier for early consultation and renewal of prescriptions, leading to improved disease control. With an aging population and increased comorbidities like psoriatic arthritis, cardiovascular conditions, and metabolic syndrome, the need for effective therapy for psoriasis is likely to increase.
Restraints
-
The high treatment costs and limited affordability remain significant restraints in the psoriasis treatment market.
Biologic treatments, including Stelara (J&J) and Cosentyx (Novartis), run up to USD 30,000–USD 80,000 per year for patients, which makes them unaffordable for most, especially in low- and middle-income nations. Poor insurance coverage and high out-of-pocket costs further limit patient access, leading many to use older, less potent drugs such as topical corticosteroids and methotrexate. Yet another principal impediment is the extended process of regulatory approvals since stringent requirements of efficacy and safety for a therapy keep delaying their market debut. The drugs, particularly the biologics, have stricter measures of pharmacovigilance imposed because they are immunosuppressant, lengthening the timeframe before their broader availability on the market. Safety concerns and side effects, as with more threats to infections and cancer risk due to biologics, further reduce patient long-term compliance. Competition from generic and biosimilar forms is also another threat to major pharma firms, as biosimilars such as Cyltezo (Boehringer Ingelheim) dampen the pricing edge of first-in-class biologics. Further, inadequate awareness and misdiagnosis in some areas stop early treatment, and hence disease progression occurs. Hospital-based administration of most injectable biologics also limits patient convenience and thereby lowers the adoption rates of those looking for self-administrative treatments.
Opportunities
-
The growing demand for novel biologic and small-molecule therapies presents significant opportunities in the psoriasis treatment market.
The surge in investment in new-generation IL and JAK inhibitors has given rise to promising products, including Bimekizumab (IL-17A/IL-17F inhibitor) and Deucravacitinib (TYK2 inhibitor), with enhanced efficacy and safety profiles. The growth in biosimilar products, including Hulio (Biocon) and Hadlima (Samsung Bioepis), is likely to push affordability and accessibility, particularly in price-sensitive markets. Also, the coupling of AI and telemedicine in dermatology is simplifying psoriasis diagnosis and treatment planning, taking the workload off the healthcare providers. Increasing demand for oral and topical agents over injectables is offering scope for drug companies to formulate more patient-acceptable therapies with higher compliance. Increasing awareness through patient support groups, social media campaigns, and educational programs for dermatologists is also increasing early diagnosis rates. With increasing incidences of psoriatic arthritis (occurring in 30% of patients with psoriasis), firms are concentrating on two-in-one drugs that treat both diseases, providing a larger market potential. Additionally, drug collaborations for combination treatments—like biologics and PDE4 inhibitors or vitamin D analogs—are providing new opportunities for effective, multi-targeted treatment modalities.
Challenges
-
One of the biggest challenges in the psoriasis treatment market is the long-term management and relapse rates associated with the disease.
Psoriasis is an autoimmune, chronic, and recurring disease, which must be treated for life, but patients often get tired of treatment and stop it because of side effects, expense, or loss of efficacy. The other issue is the absence of treatment guidelines for everyone since response to biologics is different in various patients and causes trial-and-error prescribing practices. Drug resistance and treatment failure are also issues since some patients form neutralizing antibodies against biologics, which decrease the efficacy of these drugs with time. Furthermore, although there have been drastic improvements, patient compliance rates are still low, especially for systemic treatments, as fears regarding immunosuppression and long-term side effects deter consistent use. Few clinical studies concerning pediatric psoriasis also pose issues in treatment of the condition in younger groups, limiting the access to licensed medicines. The exorbitant R&D expenses and long FDA/EMA approval processes slow down the launch of innovative drugs, restricting market growth. Another increasing issue is healthcare disparity, where access to biologic treatments is strongly biased towards developed countries, with most areas depending on outdated therapies. To overcome these challenges will need greater patient education, better drug affordability, and creative treatment approaches to facilitate continued market growth.
Psoriasis Treatment Market Segmentation Analysis
By Drugs Class
Tumor Necrosis Factor (TNF) inhibitors dominated the market with 41.4% share in 2023. Their dominance is due to their proven efficacy in controlling moderate-to-severe psoriasis, universal physician preference, and robust clinical evidence for long-term disease control. Top-selling drugs like adalimumab and etanercept remain popular because of their well-established safety profiles and extensive insurance coverage.
Interleukin (IL) inhibitors are anticipated to see the most rapid growth during the forecast period. This is because they have greater efficacy, more targeted mechanism of action, and better safety profiles than TNF inhibitors. IL-17 and IL-23 inhibitors such as secukinumab, ixekizumab, and risankizumab have been seen to be very effective in showing higher skin clearance rates, and hence, have seen greater uptake among dermatologists and patients who desire improved long-term disease control.
By Route of Administration
The parenteral segment dominated the psoriasis treatment market in 2023, mainly owing to the demand for biologic treatments, which are delivered through infusion or injection. Biologic medicines, specifically TNF inhibitors and IL inhibitors, have been established as the preferred treatment option for moderate-to-severe psoriasis patients and thus drove the dominance of this segment. The capability of such medications to yield prolonged remission along with a high level of patient compliance further established their dominance in the market.
The topical segment is anticipated to grow at the highest rate, driven by the growing need for first-line treatment among patients with mild-to-moderate psoriasis. Topical corticosteroids and vitamin D analogs continue to be most sought after due to the ease of application and the lack of systemic side effects. Furthermore, continuous developments in new topical formulations, including foam-based and combination therapies, are improving treatment outcomes and patient adherence, propelling market growth in the future.
By Distribution Channel
Hospital pharmacies held the highest market share of 40.8% in 2023. The segment's dominance is largely attributed to the high volume of biologic therapy prescriptions, which tend to be initiated and maintained in hospital environments under specialist care. Hospital pharmacies also have a significant role in offering advanced treatment for severe psoriasis cases, making high-cost drugs accessible that need professional administration.
Retail pharmacies are expected to increase at the highest rate, spurred by increasing availability of psoriasis treatments in community pharmacy outlets. The increased use of self-administered biologics, oral therapy, and topical therapy has increased prescription volumes filled in retail pharmacies. Moreover, increasing specialty pharmacy services and direct-to-patient models of distribution have improved accessibility, and retail pharmacies have emerged as a growth driver in the market.
Psoriasis Treatment Market Regional Outlook
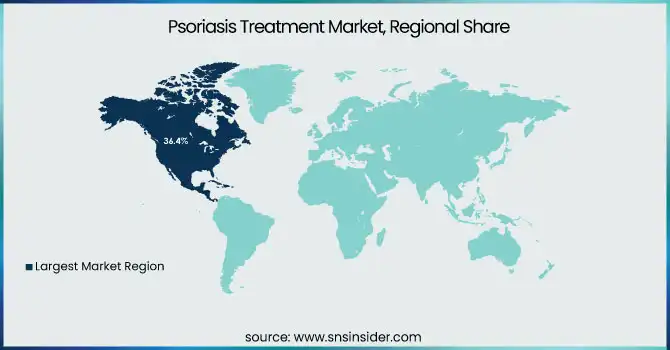
North America held the largest market share of psoriasis treatment worldwide in 2023, taking up 36.4% of the global market share. This is because the region boasts a high rate of psoriasis prevalence, augmented by robust healthcare infrastructure, friendly reimbursement policies, and extensive adoption of sophisticated biologic therapies. The availability of major pharmaceutical firms such as AbbVie, Amgen, and Johnson & Johnson has further boosted market growth through persistent product development and strategic partnerships. The increasing awareness of psoriasis as an autoimmune disease has also resulted in early detection and greater patient exposure to targeted drugs. The U.S. is still the most contributing country in North America, backed by a high prescription rate of biologics like TNF and IL inhibitors and active research and development with the aim for new treatment paradigms.
The Asia-Pacific market is anticipated to record the most rapid growth during the forecast period. The drivers of market growth here include rising prevalence of psoriasis, enhanced healthcare infrastructure, and growing patient affordability. China, India, and Japan are seeing a high rate of biologic drug uptake, facilitated by regulatory approval and increased awareness of sophisticated treatments. The rise in healthcare coverage and heightened interest in dermatology conditions are also driving the growth of the market in the region at a high rate.
Get Customized Report as per Your Business Requirement - Request For Customized Report
Key Players in the Psoriasis Treatment Market
-
AbbVie Inc. – Humira, Skyrizi
-
Amgen Inc. – Enbrel, Otezla
-
Johnson & Johnson Services, Inc. – Stelara, Tremfya
-
Novartis AG – Cosentyx
-
Eli Lilly and Company – Taltz, Olumiant
-
AstraZeneca – Saphnelo (under research for psoriasis applications)
-
Celgene Corporation (acquired by Bristol-Myers Squibb) – Otezla
-
UCB S.A. – Bimzelx
-
Merck
-
Boehringer Ingelheim International GmbH – Spesolimab (under development for psoriasis)
Recent Developments
In March 2025, Takeda focused on addressing clinical disparities in psoriasis research, highlighting the condition’s impact on over 7.5 million Americans and 60 million people globally. The company emphasized the significant comorbidities associated with psoriasis, including cardiovascular disease, psoriatic arthritis, and depression, as well as its effects on quality of life, stigmatization, and work productivity.
In Jan 2025, Biocon's shares surged by 8% following Japan's approval of its psoriasis drug, ustekinumab, for treating psoriasis vulgaris and psoriatic arthritis (PsA). The approval led to an upgrade by Jefferies, highlighting a significant market development in Biocon’s dermatology portfolio.
|
Report Attributes |
Details |
|
Market Size in 2023 |
USD 22.38 billion |
|
Market Size by 2032 |
USD 54.40 billion |
|
CAGR |
CAGR of 10.4% From 2024 to 2032 |
|
Base Year |
2023 |
|
Forecast Period |
2024-2032 |
|
Historical Data |
2020-2022 |
|
Report Scope & Coverage |
Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
|
Key Segments |
• By Drugs Class [Tumor Necrosis Factor Inhibitors, Interleukin Inhibitors, Vitamin D Analogues, Corticosteriods, Others] |
|
Regional Analysis/Coverage |
North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
|
Company Profiles |
AbbVie Inc., Amgen Inc., Johnson & Johnson Services, Inc., Novartis AG, Eli Lilly and Company, AstraZeneca, Celgene Corporation (a Bristol-Myers Squibb company), UCB S.A., Merck, Boehringer Ingelheim International GmbH. |

