Bionic Eye Market Report Scope & Overview:
Get More Information on Bionic Eye Market - Request Sample Report
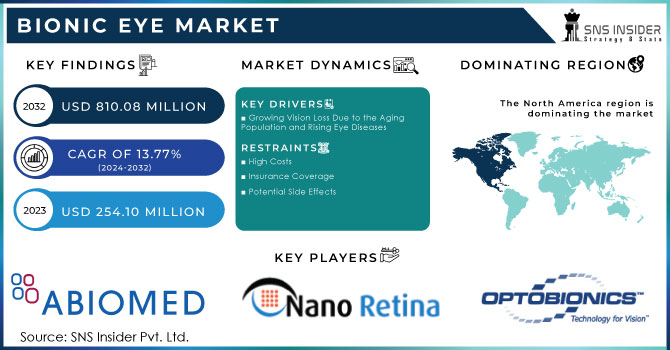
The Bionic Eye Market size was valued at USD 254.10 Million in 2023 and is expected to reach USD 810.08 Million by 2032 and grow at a CAGR of 13.77% over the forecast period 2024-2032.
The Increasing Prevalence of Vision Loss Drives Bionic Eye Market Expansion
The bionic eye market is witnessing rapid growth, driven primarily by the increasing prevalence of vision loss, particularly among aging populations. This trend is further accelerated by the rising incidence of accidents and injuries, leading to a heightened demand for sight restoration solutions. Moreover, advancements in bionic eye technology, including product approvals and intensive research and development efforts, are significantly contributing to the market's expansion. According to the World Health Organization, over 2.2 billion people worldwide were affected by vision impairment or blindness in 2023, with a significant portion suffering from unaddressed or preventable conditions. The positive outcomes of clinical trials involving bionic eyes have further accelerated their adoption among individuals with incurable eye diseases and permanent vision loss, demonstrating the safety, reliability, and long-term efficacy of this technology.
Market Insights on Vision Loss and Eye Disorders
The demand for bionic eyes is also driven by the increasing prevalence of eye disorders such as retinitis pigmentosa (RP) and age-related macular degeneration. According to the National Eye Institute, retinitis pigmentosa is a rare genetic disorder affecting approximately one in 4,000 people worldwide. Additionally, the Bright Focus Foundation reports that approximately 11 million individuals in the United States are affected by age-related macular degeneration, with this number projected to double by 2050. Globally, the foundation estimates that the number of people with macular degeneration will reach 288 million by 2040, up from 196 million in 2020.
Technological Advancements Fuel Market Growth
The bionic eye market is experiencing growth as technological advancements and research continue to evolve. The development of more affordable and accessible bionic eye solutions is driving their adoption. Additionally, rising investments in research focused on sight restoration are fueling market growth. The growing number of individuals with complete or partial blindness and the increasing prevalence of eye disorders, such as those related to diabetes, are further contributing to the demand for bionic eye technology.
Regulatory Approvals and Global Investments
Regulatory approvals and guidelines play a crucial role in ensuring the safety and efficacy of bionic eye devices. Governments worldwide are also investing in research and development to support the advancement of bionic eye technology. For instance, Bionic Vision Technologies (BVT), an Australian company, announced on January 16, 2022, that its Bionic Eye System had received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA). This designation is a significant milestone, indicating the FDA's belief that the device has the potential to treat a serious condition or fill a significant unmet medical need. This achievement marks Australia's first bionic eye system to receive such recognition from the U.S. FDA, highlighting the country's advancements in medical technology and innovation.
Innovative Electrode Implants and Bionic Lenses
The introduction of new electrode implants has the potential to provide more natural vision for patients, driving significant market growth. Additionally, the development of bionic lenses that can replace natural lenses and restore vision at all distances represents a major breakthrough. However, while the bionic eye market is growing, it faces challenges such as the high costs associated with these devices, which can limit accessibility. Potential side effects, such as skin reactions, may also hinder adoption. Nevertheless, ongoing research and technological advancements are addressing these challenges, creating new opportunities for market expansion. As research continues and technology improves, bionic eyes have the potential to transform the lives of millions of people with vision impairment.
Market Dynamics
Drivers
-
Growing Vision Loss Due to the Aging Population and Rising Eye Diseases:
The increasing prevalence of vision loss, driven by an aging population and rising incidence of eye diseases, is a primary driver. Technological advancements, such as improved electrode implants and the development of bionic lenses, are making these devices more effective and accessible. Positive outcomes from clinical trials have demonstrated the safety, reliability, and efficacy of bionic eyes, boosting their adoption. Government support, including favorable regulations and policies, is fostering market growth by facilitating the development and approval of these devices. Investments in research and development are contributing to market innovation and expansion by driving advancements in bionic eye technology. Rising awareness among patients and healthcare providers is also driving demand, as more individuals become aware of the potential benefits of these devices. Ultimately, the ability of bionic eyes to restore vision and improve the quality of life for individuals with vision impairment is a significant driver for the market.
Restraints
-
High Costs:
The high cost associated with bionic eye devices can limit accessibility, particularly for individuals with limited financial resources.
-
Insurance Coverage:
Many health insurance plans do not fully cover the cost of bionic eye procedures, making them unaffordable for some patients.
-
Potential Side Effects:
Bionic eye implants may be associated with potential side effects, such as skin reactions or discomfort, which could deter some individuals from considering the procedure.
Key Segmentation
By Type
The external eye segment dominated the bionic eye market, capturing over 60% of the revenue in 2023. This dominance stems from its ability to enhance visual experiences. Market players are actively investing in technological advancements to improve patient outcomes, further solidifying the segment's position. The external eye segment is poised for the fastest growth, projected to expand at a CAGR of 13.6% during the forecast period. This rapid growth is attributed to several factors. Firstly, the segment offers superior visual experiences compared to other options. Secondly, ongoing technological innovations from various industry competitors are driving down procedure costs, making them more accessible to a wider patient population. These combined factors are expected to fuel significant market expansion in the coming years.
By End-use
The hospital segment dominated the bionic eye market, accounting for approximately 45% of the revenue in 2023, and is expected to maintain its lead throughout the forecast period. Hospitals provide the initial point of care, staffed with experienced ophthalmologists and advanced equipment to ensure efficient patient treatment. Expanding medical coverage and successful surgical outcomes are driving an increase in surgical procedures, contributing to the segment's growth. Meanwhile, the outpatient facilities segment is poised for the fastest growth, projected to expand at a CAGR of 13.7%. This surge is attributed to a growing preference for outpatient settings over hospitals. Collaborations between hospitals and product developers for clinical trials have significantly accelerated the segment's development. These factors collectively position outpatient facilities as a rapidly expanding segment within the market.
Regional Analysis
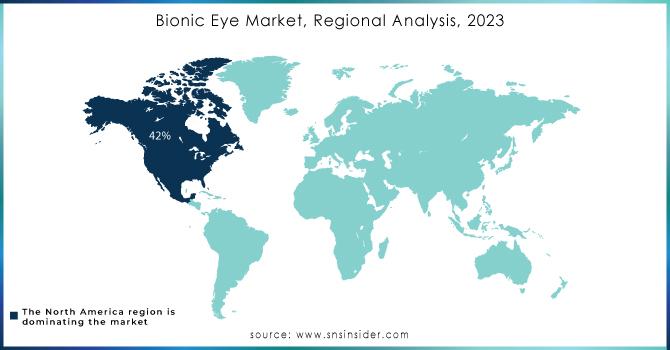
North America dominated the bionic eye market, capturing approximately 42% of the market share in 2023, and is expected to maintain its lead throughout the forecast period. The region's favorable reimbursement policies, well-developed healthcare infrastructure, and robust research activities contribute to its dominance. Technological advancements, such as overcoming the challenges of printed electronics on curved surfaces, are driving significant market growth. In 2018, a collaborative effort between researchers from Stanford University and Israel, funded by the U.S. National Institutes of Health and the U.S. Air Force, focused on developing a solar-powered retina to treat blindness caused by retinitis pigmentosa.
Asia Pacific is poised for the fastest growth, projected to expand at a CAGR of 15.5% during the forecast period. The region's rapid growth is fueled by the accelerated pace at which companies are introducing innovative bionic eye implants. In January 2020, Bionic Vision Technologies, Australia, announced the results of a pilot study conducted on patients with late-stage retinitis pigmentosa using its Gen3 bionic eye system.
Need any customization research on Bionic Eye Market - Enquiry Now
Key Players
The Major Players are Optobionics Corporation, Monash Vision Group, Nano Retina Ltd., ABIOMED, AAV Media, LLC., NEOSTRATA, Nidek Co. Ltd., Integrated Bionic Microsystems Laboratory, Pixium Vision, Second Sight Medical Products LLC, Biomedical Technologies S.L., MetaModal LLC, THE BIONIC EYE, Bionic Vision Technologies, and others playres
Recent Developments
-
Second Sight Medical Products:
-
March 2024: Announced the launch of the Orion Visual Cortical Prosthesis System, designed to enhance visual perception in patients with severe vision loss.
-
-
Bionic Vision Technologies:
-
June 2024: Revealed advancements in their bionic eye prototype, featuring improved electrode implants for better visual acuity and stability.
-
-
Pixium Vision:
-
April 2024: Released updated clinical trial results showing significant improvements in visual outcomes with their PRIMA bionic vision system.
-
-
Eyre Bioengineering:
-
July 2024: Introduced a new generation of bionic lenses, incorporating advanced materials for greater comfort and clarity.
-
-
Implantable Bionic Eyes Inc.:
-
February 2024: Completed a successful pilot study for their next-generation bionic eye device, demonstrating enhanced functionality and user satisfaction.
-
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 254.10 million |
| Market Size by 2032 | US$ 810.08 million |
| CAGR | CAGR of 13.77% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Type (External Eye, Implanted Eye) • By End-use (Hospitals, Outpatient Facilities, Research and Manufacturing) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Optobionics Corporation, Monash Vision Group, Nano Retina Ltd., ABIOMED, AAV Media, LLC., NEOSTRATA, Nidek Co. Ltd., Integrated Bionic Microsystems Laboratory, Pixium Vision, Second Sight Medical Products LLC, Biomedical Technologies S.L., MetaModal LLC, THE BIONIC EYE, Bionic Vision Technologies and others. |
| Key Drivers | • Growing Vision Loss Due to the Aging Population and Rising Eye Diseases Fuels the Bionic Eyes Market |
| Restraints | • The high cost associated with bionic eye devices can limit accessibility, particularly for individuals with limited financial resources. • Many health insurance plans do not fully cover the cost of bionic eye procedures, making them unaffordable for some patients. • Bionic eye implants may be associated with potential side effects, such as skin reactions or discomfort, which could deter some individuals from considering the procedure. |

