Deep Brain Stimulation (DBS) Devices Market Report Scope & Overview:
To Get More Information on Deep Brain Stimulation (DBS) Devices Market - Request Sample Report
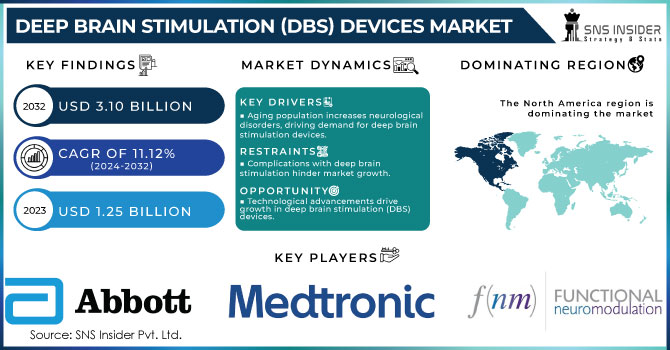
The Deep Brain Stimulation (DBS) Devices Market Size was valued at USD 1.25 billion in 2023 and is witnessed to reach USD 3.10 billion by 2032 and grow at a CAGR of 11.12% over the forecast period 2024-2032.
The high mortality and disease burden, coupled with the lack of effective pharmacotherapies necessitates the development and utilization of long-term solutions such as deep brain stimulation. In addition, the rising interest in large neurological disease burden has triggered demand for deep brain stimulators as a substitute therapy.
Also, technological improvements in deep brain stimulation devices are expected to offer lucrative growth opportunities. Examples of these technological advances include multi-target stimulation, robotic implantation-assistance systems microelectrode designs, rechargeable implantable pulse generators, as well as personalized directed programming. For example, in July 2022 Abbott announced FDA Breakthrough Device Designation for its deep brain stimulation system. The designation is awarded to study the use of the DBS system for treatment-resistant depression, an advanced manifestation of major depressive disorder.
This recognition highlights the significance of Abbott's novel path to helping patients with a challenging and serious medical condition, advancing the opportunities for breakthroughs in depression treatment. The occurrence of mobility and psychiatric conditions is very high in the aging population, particularly in countries such as the U.S., and Canada where the geriatric prevalence is increasing rapidly. Symptoms of Parkinson's Disease, for example, occur typically around the age of 60 on average (Parkinson's Foundation). The elderly population suffers from these disorders the most. Likewise, the Canadian Psychological Association reports that 2% of Canadians have obsessive-compulsive disorder.
All, these factors are skyrocketing the growth of the deep brain stimulation devices market growth.
.MARKET DYNAMICS:
KEY DRIVERS:
- Rapidly Aging Population is responsible for rising neurological disorders which is Generating the Need for Deep Brain Stimulation Devices
- Surging Demand for Deep Brain Stimulation Devices because of the Rising Popularity of Minimally Invasive Procedures.
RESTRAINTS:
- Complications regarding Deep Brain Stimulation Procedures are Hindering the Growth of the Market.
- Implementing Stringent Government Policies Can Limit the Adoption of Deep Brain Stimulation (DBS) Devices.
OPPORTUNITY:
- Technological Advancements are Offering a Lucrative Growth Opportunity for Deep Brain Stimulation (DBS) Devices Systems.
- Investment in R&D is Responsible for the Deep Brain Stimulation (DBS) Devices System Market Growth During Upcoming Years.
KEY MARKET SEGMENTATION:
By Product
- Single Channel
- Dual Channel
Product-wise the market is divided into single-channel and dual-channel DBS devices. With a 58% market share in 2023, the dual-channel segment had dominated due to its widespread application across various surgical procedures. Dual-channel devices are among the safest and most effective-in-use tools used in surgical procedures. As a result, the burgeoning occurrence rate of disabling neurological diseases, the mounting need for surgeries related to Parkinson's disease (PD), and the mushrooming number of hospitals exchanging single-channel deep brain stimulation (DBS) devices with dual-channel ones anticipating advantages such as elevated treatment outcomes are some crucial factors aiding in favoring growth trajectory across this segment. In addition, the continued advancements in technology and new product offerings are driving growth within this sector. Single-channel DBS Devices are expected to witness the highest growth rate from 2023 to 2030.
By Application
- Pain Management
- Epilepsy
- Essential Tremor
- Obsessive Compulsive Disorder (OCD)
- Depression
- Dystonia
- Parkinson’s Disease
- Others
Factors such as the application; the Parkinson's disease accounted for a 66.01 % share in 2023. The growth is credited to a surge in the rate of approvals by the U.S. FDA for deep brain stimulation therapy, the high prevalence of Parkinson's Disease internationally, and enhanced research & development activities. In March 2023, for example, a team at Michigan Technological University reported using neuromorphic computing to increase the efficiency by which deep brain stimulation (DBS) improves Parkinson's disease. The new model could lead this whole category to step-change growth in the future.
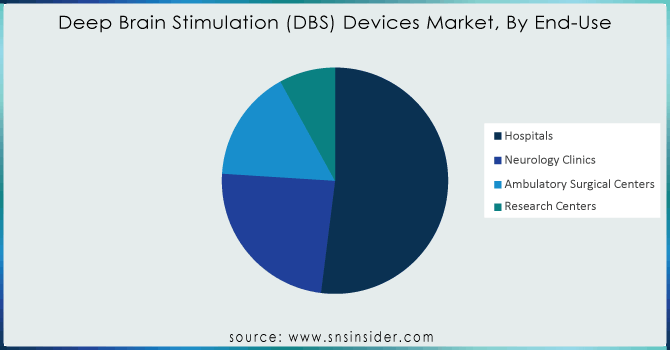
By End-Use
- Hospitals
- Neurology Clinics
- Ambulatory Surgical Centers
- Research Centers
Overall, the market was led by the hospital end-use segment accounting for a share of 52% in 2023. In hospital settings and with the increasing prevalence of Parkinson's disease, essential tremor is expected to expand more rapidly due in large part to hospitals & growing number will materialize during complex surgeries for medication-resistant treatment upsurge. Furthermore, the demand for technologically advanced DBS devices along with encouraging reimbursement policies is expected to drive significant growth in this segment over the forecast years. Medtronic is a prime illustrative example of providing comprehensive services for ensuring ongoing coverage, as well as ease of payment with DBS (of any variety) across the spectrum.
Do You Need any Customization Research on Deep Brain Stimulation (DBS) Devices Market - Enquire Now
REGIONAL COVERAGE:
- North America
- US
- Canada
- Mexico
- Europe
- Eastern Europe
- Poland
- Romania
- Hungary
- Turkey
- Rest of Eastern Europe
- Western Europe
- Germany
- France
- UK
- Italy
- Spain
- Netherlands
- Switzerland
- Austria
- Rest of Western Europe
- Eastern Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Vietnam
- Singapore
- Australia
- Rest of Asia Pacific
- Middle East & Africa
- Middle East
- UAE
- Egypt
- Saudi Arabia
- Qatar
- Rest of Middle East
- Africa
- Nigeria
- South Africa
- Rest of Africa
- Middle East
- Latin America
- Brazil
- Argentina
- Colombia
- Rest of Latin America
REGIONAL ANALYSES
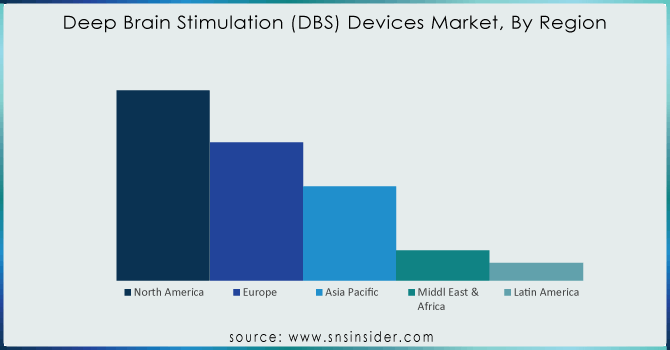
In 2023, the market is dominated by North America holding a share of 52.3% due to an increase in government funding and initiatives taken to create awareness about Parkinson's disease will drive demand for deep brain stimulator devices Additionally, the market growth in this region is driven by the presence of key players along with availability of advanced healthcare infrastructure and favorable government initiatives. The National Institute of Neurological Disorders and Stroke, for example, funds research activities aimed at assessing both the safety and efficacy of deep brain stimulation devices used in treating Parkinson's disease.
Additionally, the Asia Pacific is projected to grow passively during the projected timeframe. It is because of the increasing prevalence rate for neurodegenerative disorders which may result in increased demand for advanced and long-term treatments. Moreover, increasing patient base coupled with rising awareness regarding the availability of treatment for neurological disease and advances in the clinical development environment within developing countries is anticipated to boost deep brain stimulation devices market growth over the forecast period. In addition, the availability of lucrative growth opportunities in emerging economies such as Japan India, and China among others is expected to drive the deep brain stimulation devices market. Rise in the establishment of organizations such as the Asia Pacific Centre for Neuromodulation which is found in Tugun Australia to research and create awareness regarding DBS, this will support deep brain stimulation devices market growth.
Key Players
The deep brain stimulation devices market players include Abbott Laboratories, Beijing Pinchi Medical Equipment Co., Ltd., Boston Scientific Corporation, Fisher Wallace Laboratories, Functional Neuromodulation Ltd., Medtronic, NeuroPace Inc., Renishaw PLC, Aleva Neurotherapeutics S.A. & LivaNova PLC & Other Players.
RECENT DEVELOPMENTS
- In November 2023, Nexstim announced a collaboration with Otsuka Pharmaceutical to Utilize brain stimulation technology in neurological and psychiatric disorders.
- In September 2023, Neuropace initiated a clinical trial to assess use for Parkinson's moving the potential market options of DBS.
- In AUGUST 2023, The Phase IIB, double-blind ACHIEVE study demonstrates Percept PC+ as an effective option for the treatment of Tourette Syndrome.
- In May 2023, Abbott's MRI-Conditional, Rechargeable Intellineura DBS System for Parkinson's and Essential Tremor received FDA clearance.
- In February 2023, Aleva Neurotherapeutics secured €40M to develop its closed-loop DBS system for Parkinson's, which is using real-time brain activity monitoring.
- In January 2023, the Percept PC+ DBS system from Medtronic - commercially available in Europe - received first-of-its-kind approval for integrated directional steering to ensure accurate targeting of brain regions.
| Report Attributes | Details |
| Market Size in 2023 | US$ 1.25 Billion |
| Market Size by 2032 | US$ 3.10 Billion |
| CAGR | CAGR of 11.12% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | •By Product (Single-channel, Dual-channel) •By Application (Parkinson’s Disease, Epilepsy, Essential Tremor, Obsessive Compulsive Disorder (OCD), Depression, Dystonia, Parkinson’s Disease & Others) •By End-use (Hospitals, Neurology Clinics, Ambulatory Surgical Centers & Research Centers) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Abbott Laboratories, Beijing Pinchi Medical Equipment Co., Ltd., Boston Scientific Corporation, Fisher Wallace Laboratories, Functional Neuromodulation Ltd., Medtronic, NeuroPace Inc., Renishaw PLC, Aleva Neurotherapeutics S.A. & LivaNova PLC & Other Players |
| Key Drivers | •Rapidly Aging Population is responsible for rising neurological disorders which is Generating the Need for Deep Brain Stimulation Devices •Surging Demand for Deep Brain Stimulation Devices because of the Rising Popularity of Minimally Invasive Procedures. |
| RESTRAINTS | •Complications regarding Deep Brain Stimulation Procedures are Hindering the Growth of the Market. •Implementing Stringent Government Policies Can Limit the Adoption of Deep Brain Stimulation (DBS) Devices. |

