Pulmonary Function Testing Market Report Scope & Overview:
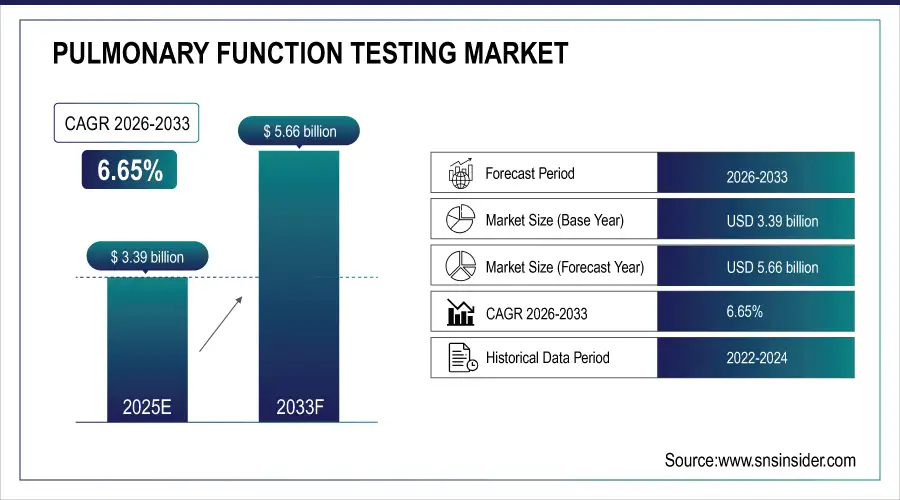
The Pulmonary Function Testing Market size was valued at USD 3.39 Billion in 2025E and is projected to reach USD 5.66 Billion by 2033, growing at a CAGR of 6.65% during 2026-2033.
The Pulmonary Function Testing Market analysis highlights the increasing prevalence of respiratory diseases, growing adoption of advanced diagnostic technologies, rising demand for portable and home-based pulmonary function testing devices, and expanding investments in research and development.
By 2025, more than 60% of hospitals in high-income countries upgraded to digital spirometers with AI-assisted interpretation, improving diagnostic accuracy and workflow efficiency for lung function assessment.
Market Size and Forecast:
-
Market Size in 2025E: USD 3.39 Billion
-
Market Size by 2033: USD 5.66 Billion
-
CAGR: 6.65% from 2026 to 2033
-
Base Year: 2025
-
Forecast Period: 2026–2033
-
Historical Data: 2022–2024
To Get more information on Pulmonary Function Testing Market - Request Free Sample Report
Pulmonary Function Testing Market Trends
-
Growing adoption of portable and handheld PFT devices enables home-based monitoring, improving accessibility and early detection of respiratory diseases.
-
Integration of digital health technologies and cloud-based data management enhances remote patient monitoring and real-time analysis of pulmonary function results.
-
Increasing prevalence of chronic respiratory diseases, such as COPD and asthma, drives demand for advanced diagnostics and preventive healthcare solutions.
-
Rising collaborations between hospitals, clinics, and PFT device manufacturers accelerate development of innovative, user-friendly testing equipment and software solutions.
-
Expansion of pediatric and geriatric PFT testing reflects growing awareness, early diagnosis initiatives, and focus on age-specific respiratory healthcare needs.
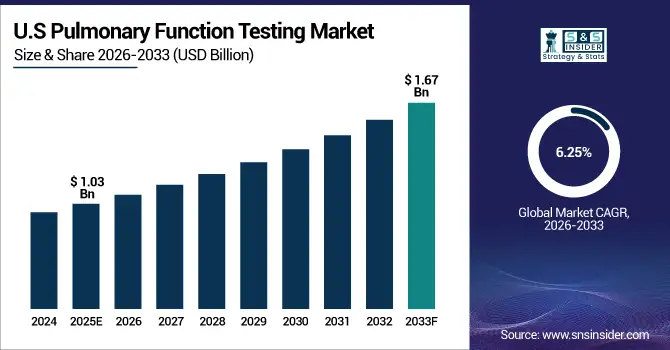
The U.S. Pulmonary Function Testing Market size was valued at USD 1.03 Billion in 2025E and is projected to reach USD 1.67 Billion by 2033, growing at a CAGR of 6.25% during 2026-2033. Pulmonary Function Testing Market growth is driven by rising prevalence of respiratory diseases, technological advancements in portable and automated PFT devices, and increased adoption of home-based testing. Hospitals and specialty clinics dominate the market, while digital integration and growing pediatric and geriatric testing demand drive rapid growth and innovation.
Pulmonary Function Testing Market Growth Drivers:
-
Rising Prevalence of Chronic Respiratory Diseases and Increasing Demand for Advanced Diagnostic Tools
The Pulmonary Function Testing (PFT) Market is primarily driven by the increasing prevalence of chronic respiratory diseases such as COPD, asthma, and interstitial lung disorders. Growing awareness about early diagnosis, preventive healthcare, and monitoring disease progression is encouraging the adoption of advanced PFT devices. Additionally, the demand for portable, automated, and home-based testing solutions is boosting market growth. Rising investments in R&D, technological advancements, and hospital and clinic infrastructure expansion further propel the market globally.
Over 65% of national respiratory guidelines updated in 2024–2025 mandated spirometry for early detection in at-risk adults, significantly increasing PFT utilization in primary care and screening programs.
Pulmonary Function Testing Market Restraints:
-
High Cost of Pulmonary Function Testing Equipment Limiting Adoption in Emerging Economies
The high cost of advanced pulmonary function testing devices, including spirometers, plethysmographs, and impulse oscillometry systems, remains a significant market restraint. Expensive maintenance, calibration requirements, and the need for trained personnel further limit adoption, especially in emerging economies. Budget constraints in smaller hospitals and clinics restrict large-scale deployment. Additionally, reimbursement issues and inconsistent insurance coverage hinder widespread utilization. These financial and operational challenges slow market penetration, particularly in regions with limited healthcare infrastructure, reducing the overall growth potential despite rising demand.
Pulmonary Function Testing Market Opportunities:
-
Growing Adoption of Home-Based and Portable Pulmonary Function Testing Solutions
The increasing adoption of portable and home-based pulmonary function testing devices presents a major growth opportunity. Patients with chronic respiratory diseases prefer convenient, remote monitoring solutions, driving demand for wearable and connected testing devices. Integration with digital health platforms, telemedicine, and cloud-based data management enables real-time tracking and better patient management. Expanding awareness among pediatric and geriatric populations, combined with rising investments in affordable portable devices, creates potential for market expansion across both developed and emerging regions, offering long-term growth prospects for manufacturers.
By 2025, nearly 60% of COPD and asthma patients expressed a strong preference for at-home lung function testing to avoid frequent clinic visits and enable daily symptom tracking.
Pulmonary Function Testing Market Segment Analysis
-
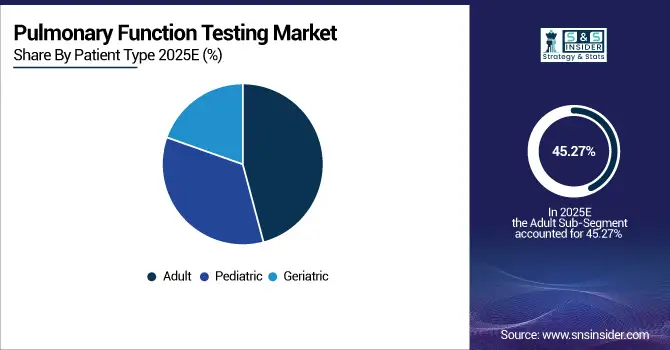
By patient type, adults led the market with a 45.27% share in 2025, while the pediatric segment is the fastest growing, registering a CAGR of 8.10%.
-
By application, obstructive lung diseases dominated with 61.03% share in 2025, whereas restrictive lung diseases are growing the fastest at a CAGR of 5.27%.
-
By type, hardware led the market with 45.19% share in 2025, while services are the fastest-growing segment, recording a CAGR of 5.42%.
-
By end-user, hospitals held 67.51% share in 2025, while home care settings are growing the fastest with a CAGR of 6.50%.
By Patient Type, Adult Leads Market While Pediatric Registers Fastest Growth
In 2025, the adult segment dominates the pulmonary function testing market due to the high prevalence of chronic respiratory diseases such as COPD and asthma in adults. Hospitals and clinics frequently perform routine pulmonary assessments for early diagnosis and disease management. Meanwhile, the pediatric segment is registering the fastest growth, driven by increasing awareness of childhood respiratory conditions, government screening programs, and rising adoption of portable and home-based PFT devices for children, ensuring accurate monitoring from an early age.
By Application, Obstructive Lung Disease Dominate While Restrictive Lung Disease Shows Rapid Growth
Obstructive lung diseases, including COPD, asthma, and bronchiectasis, dominate the market, accounting for the majority of testing procedures in hospitals and specialty clinics. The increasing prevalence of these disorders and the emphasis on early diagnosis drive high demand. However, restrictive lung diseases are showing rapid growth, attributed to rising incidences of interstitial lung disorders, fibrosis, and other pulmonary restrictions. Technological advancements in pulmonary function testing devices now allow accurate assessment of both obstructive and restrictive conditions, fueling segment growth.
By Type, Hardware Lead While Services Registers Fastest Growth
By types, hardware dominates the pulmonary function testing market, as traditional spirometers, plethysmographs, and gas analyzers form the core of clinical diagnostics. Hospitals and specialty clinics rely heavily on these instruments for accurate patient assessment. Meanwhile, the services segment is growing rapidly, including calibration, maintenance, training, and cloud-based analytics. The increasing adoption of outsourced services by healthcare providers to reduce operational burden and ensure device accuracy is accelerating the growth of service-based offerings within the market.
By End-User, Hospital Lead While Home Care Settings Grow Fastest
Hospitals remain the leading end-user in the pulmonary function testing market, as they handle the majority of respiratory diagnostic procedures due to high patient volumes and advanced infrastructure. Specialty clinics and diagnostic laboratories support additional testing demand. However, home care settings are growing the fastest, driven by the rising popularity of portable pulmonary function testing devices, telemedicine integration, and remote patient monitoring initiatives. Patients increasingly prefer home-based solutions for convenience, frequent testing, and disease management without hospital visits.
Pulmonary Function Testing Market Regional Analysis:
North America Pulmonary Function Testing Market Insights
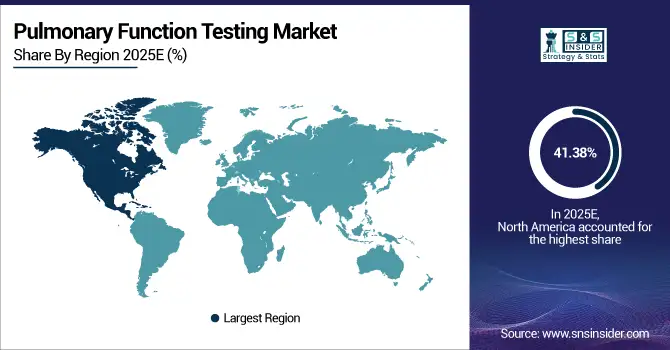
In 2025 North America dominated the pulmonary function testing market and accounted for 41.38% of revenue share, this leadership is due to the advanced healthcare infrastructure. High prevalence of chronic respiratory diseases like COPD and asthma drives demand. Hospitals and specialty clinics adopt advanced hardware and digital PFT solutions. Home care and portable testing devices are increasingly utilized. Continuous R&D and strong reimbursement policies further support market growth.
Get Customized Report as per Your Business Requirement - Enquiry Now
U.S. Pulmonary Function Testing Market Insights
The U.S. pulmonary function testing market is expanding steadily with hospitals dominating end-user adoption. Advanced technological integration, including cloud-based and digital solutions, boosts testing efficiency. Rising pediatric and geriatric testing demand supports market growth. Home-based and portable devices are gaining traction among patients.
Asia-pacific Pulmonary Function Testing Market Insights
Asia-pacific is expected to witness the fastest growth in the pulmonary function testing market over 2026-2033, with a projected CAGR of 7.27% due to rising respiratory disease prevalence and growing healthcare infrastructure. Increasing awareness about early diagnosis and preventive care drives adoption. Government initiatives for chronic disease management further support market growth. The demand for portable and home-based PFT devices is accelerating. Rapid urbanization and increasing disposable incomes in countries like India and Japan also boost market opportunities.
China Pulmonary Function Testing Market Insights
China dominates the pulmonary function testing market in Asia-Pacific, driven by high COPD and asthma prevalence. Expansion of hospitals and specialty clinics enhances device penetration. Increasing healthcare spending supports adoption of advanced hardware and service offerings.
Europe Pulmonary Function Testing Market Insights
In 2025, Europe shows significant adoption of pulmonary function testing devices due to high respiratory disease burden. Germany leads the region, supported by advanced hospitals and clinics. Increasing use of portable and connected pulmonary function testing devices is observed. Preventive healthcare initiatives and chronic disease awareness programs boost market penetration. Rising R&D and collaborations with device manufacturers contribute to innovation and growth.
Germany Pulmonary Function Testing Market Insights
Germany dominates Europe’s pulmonary function testing market with strong hospital networks and advanced diagnostic capabilities. Increasing prevalence of obstructive and restrictive lung diseases drives demand. Home care adoption of portable devices is accelerating. Government initiatives and health insurance coverage support accessibility.
Latin America (LATAM) and Middle East & Africa (MEA) Pulmonary Function Testing Market Insights
The Pulmonary Function Testing Market is experiencing moderate growth in the Latin America (LATAM) and Middle East & Africa (MEA) regions, due to the rising awareness and improving healthcare infrastructure. Portable pulmonary function testing devices support testing in remote areas. Limited access to advanced hospitals restrains growth but presents opportunities for home-based solutions. Increasing government initiatives for respiratory health support adoption. Expansion of clinics and collaborations with international device manufacturers boost market penetration.
Pulmonary Function Testing Market Competitive Landscape:
ZOLL Medical Corporation focuses on advanced respiratory monitoring devices and life-support solutions. Its pulmonary function testing offerings are integrated with critical care and emergency equipment, enhancing hospital workflow efficiency. The company emphasizes R&D for innovative diagnostics, portable PFT solutions, and digital integration to enable real-time monitoring, improving patient management and early detection of respiratory conditions
-
In May 2025, ZOLL introduced the use of z-scores in pulmonary function testing, offering a new and improved way to measure test results. This advancement enhances the accuracy and reliability of PFTs, contributing to better patient outcomes.
CAIRE, Inc. specializes in respiratory devices, including PFT hardware for clinical and home settings. Its offerings emphasize portability, ease-of-use, and integration with patient monitoring platforms. The company invests in R&D to improve device accuracy, patient compliance, and service offerings. Expansion into emerging markets and home-based diagnostics drives growth.
-
In February 2025, CAIRE, Inc. expanded its services by adding Accurate Biomed Services to its authorized service network, aiming to enhance support for medical equipment providers and oxygen therapy patients in the U.S.
COSMED srl provides advanced cardiopulmonary diagnostic systems, including PFT devices for obstructive and restrictive lung diseases. Its solutions focus on accuracy, compact design, and digital data management. The company targets hospitals, clinics, and research institutions while investing in R&D to enhance usability and expand adoption of portable and home-based testing systems globally.
-
In October 2024, COSMED srl launched the second generation of its OMNIA Software, a comprehensive data management platform for metabolic, lung function, and body composition assessments, enhancing user interaction with COSMED products.
Pulmonary Function Testing Market Key Players:
Some of the Pulmonary Function Testing Market Companies are:
-
ZOLL Medical Corporation
-
Teleflex Incorporated
-
CAIRE, Inc.
-
COSMED srl
-
Schiller AG
-
GANSHORN Medizin Electronic GmbH
-
PulmOne Advanced Medical Devices
-
ndd Medical Technologies
-
KoKo PFT
-
Geratherm Medical AG
-
ECO MEDICS AG
-
Morgan Scientific
-
SIBEL, S.A.U.
-
Clarity Medical
-
Data Sciences International
-
IngMar Medical
-
JK Medical System
-
MEC COMPANY LTD
-
Medline Industries, LP
-
Recorders and Medicare Systems
| Report Attributes | Details |
|---|---|
| Market Size in 2025E | USD 3.39 Billion |
| Market Size by 2033 | USD 5.66 Billion |
| CAGR | CAGR of 6.65 % From 2026 to 2033 |
| Base Year | 2025E |
| Forecast Period | 2026-2033 |
| Historical Data | 2022-2024 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Patient Type (Pediatric, Adult, and Geriatric) • By Application (Obstructive Lung Disease and Restrictive Lung Disease) • By Type (Hardware, Services, and Software) • By End-User (Hospital, Specialty Clinics, Diagnostics Laboratories, Home Care Settings, and Others) |
| Regional Analysis/Coverage | North America (US, Canada), Europe (Germany, UK, France, Italy, Spain, Russia, Poland, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Australia, ASEAN Countries, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Mexico, Colombia, Rest of Latin America). |
| Company Profiles | ZOLL Medical Corporation, Teleflex Incorporated, CAIRE, Inc., COSMED srl, Schiller AG, GANSHORN Medizin Electronic GmbH, PulmOne Advanced Medical Devices, ndd Medical Technologies, KoKo PFT, Geratherm Medical AG, ECO MEDICS AG, Morgan Scientific, SIBEL, S.A.U., Clarity Medical, Data Sciences International, IngMar Medical, JK Medical System, MEC COMPANY LTD, Medline Industries, LP, Recorders and Medicare Systems |