Scleroderma Therapeutics Market Report Size Analysis:
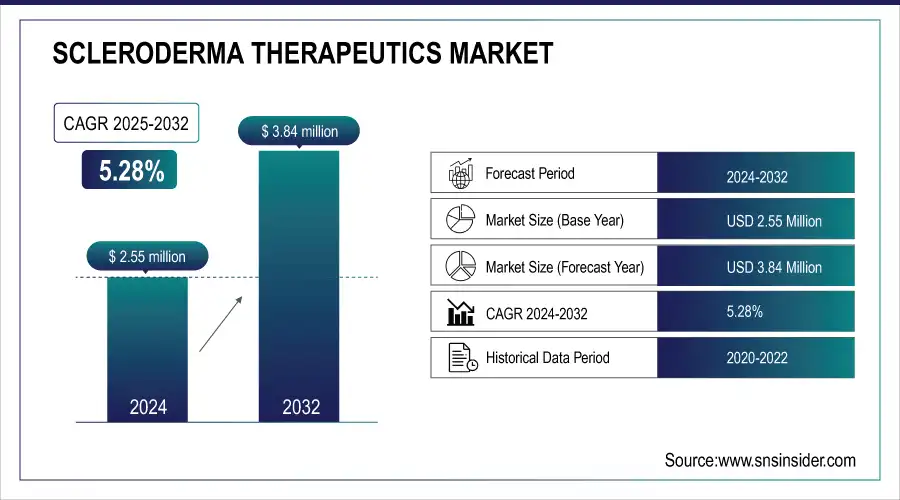
The Scleroderma Therapeutics Market size was valued at USD 2.55 million in 2024 and is expected to reach USD 3.84 million by 2032, growing at a CAGR of 5.28% over the forecast period of 2025-2032.
Driven by the rising frequency of autoimmune diseases, including systemic sclerosis and localized scleroderma, which are complicated connective tissue disorders marked by skin fibrosis, interstitial lung disease (ILD), and pulmonary arterial hypertension (PAH), the Scleroderma Therapeutics market is seeing a steady increase.
To Get more information on Scleroderma Therapeutics Market - Request Free Sample Report
The U.S. National Institutes of Health (NIH) estimates that scleroderma impacted around 300,000 Americans in 2023; most of the cases are related to systemic sclerosis. Supported by robust government financing and regulatory incentives, including orphan drug designations for new treatments, the U.S. Scleroderma Therapeutics market had a notable share in 2023 and generated sales over USD 1 billion. While increased research grants have strengthened the scleroderma therapeutic pipeline, government bodies, including the FDA, have sped approvals of novel FDA-approved medications for scleroderma. These elements together help to explain the increasing Scleroderma Therapeutics market size as well as highlight good Scleroderma Therapeutics market expansion and changing Scleroderma Therapeutics market trends globally.
Advances in immunosuppressive therapy for scleroderma, biologics for scleroderma, and new treatments aiming at the many pathogeneses of this autoimmune connective tissue disease drive the global scleroderma therapeutics market. Particularly for systemic sclerosis therapeutics, government regulatory agencies around the world have expanded financing and simplified clinical trial approvals to hasten the discovery of disease-modifying therapies. Innovation has been sparked by the orphan drug designations and fast-track approvals for medications aiming at skin fibrosis, ILD, and PAH associated with scleroderma by the U.S. FDA. Furthermore, helping patient outcomes are digital health and AI-driven diagnostic tools, enhancing early identification and tailored treatment options. Together with increasing awareness and better epidemiological data collection by government health organizations, these advancements are influencing the Scleroderma Therapeutics market study and encouraging continuous Scleroderma Therapeutics market growth.
Scleroderma Therapeutics Market Dynamics:
Drivers
-
Rising Scleroderma Incidence and Awareness Affecting Therapeutic Demand
Key drivers of the demand for therapies worldwide include the increasing incidence of scleroderma and greater awareness of it. With an incidence rate of roughly 8.64 cases per 100,000 person-years and a worldwide prevalence of roughly 18.87 instances per 100,000 persons, recent epidemiological statistics show that systemic sclerosis (SSc), a main kind of scleroderma, is rather common. This corresponds to around 670,000 new diagnoses annually worldwide, mostly affecting adults in high-income nations and women. Early detection and treatment, starting from better diagnostic criteria and raised awareness among healthcare providers, have helped to increase the patient count seeking therapies.
Government and non-profit projects include the FDA clearance of the Scleroderma Research Foundation's CONQUEST clinical trial platform in late 2023 drive research and development of new medicines even more. Treating possibilities are being expanded by pharmaceutical partnerships and the launch of creative therapeutics including stem cell and gene therapies. By attending to unmet medical requirements and motivating more patients to acquire sophisticated therapies for better disease management, these elements together enhance Scleroderma Therapeutics market growth.
Restraints
-
High Treatment Costs and Legal Obstacles Limit Market Accessibility
The great cost of treatment and strict regulatory rules that restrict patient access and postpone drug availability provide major obstacles for the scleroderma therapeutic industry. Particularly in low- and middle-income nations, advanced medicines include biologics and tailored immunomodulatory medications that can have exorbitant cost tags, which limit general adoption. Furthermore, complicating the regulatory scene for drug approval are extensive, expensive clinical trials and compliance procedures, which delay the launch of novel therapies onto the market. This limits therapy choices that reach patients quickly, even with rising disease frequency.
Because scleroderma is chronic and calls for long-term treatment, individuals' and healthcare systems' financial load is further taxed. Therefore, by limiting pricing and accessibility, these economic and legal obstacles impede the growth of the market and thereby constrain the possible advantages of new treatments for many people worldwide.
Scleroderma Therapeutics Market Segmentation Analysis:
By Indication
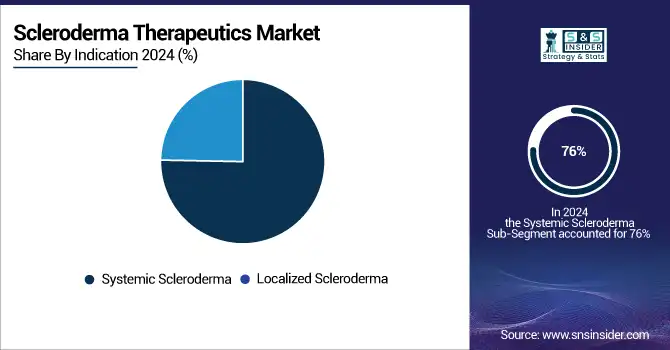
Driven by its greater frequency and severe internal organ involvement, including skin fibrosis and interstitial lung disease, systemic scleroderma accounted for the largest Scleroderma Therapeutics market share of 76% in 2024 within the global Scleroderma Therapeutics market. Rising incidence rates, particularly in North America and Europe, confirmed by government epidemiological data, call for more funding for clinical trials of systemic sclerosis therapies. For instance, the NIH and European Commission have given systemic sclerosis top priority on their autoimmune disease research agendas, therefore enabling trials of new biologics and cell treatments.
On the other hand, because of better diagnosis distinction and growing knowledge of the effect of this subtype on skin integrity, localized scleroderma drugs are projected to develop at the fastest CAGR. Particularly in dermatology offices, government health initiatives supporting early identification and treatment access have broadened the patient pool for localized scleroderma. Supported by orphan drug designations and regulatory incentives, emerging therapies, including topical immunosuppressants and biologics, are becoming popular. Targeted localized scleroderma treatments are being developed faster because in great part due to advances in personalized medicine and genomic research supported by public health organizations.
By Drug Class
With a 28% of Scleroderma Therapeutics market share in 2024, the immunosuppressors segment dominated, demonstrating its vital importance in controlling systemic sclerosis and related consequences, such as Raynaud's phenomenon and interstitial lung disease. Supported by continuous clinical trials sponsored by government grants, particularly NIH-sponsored studies, drugs, including mycophenolate mofetil and cyclophosphamide, remain cornerstones of immunosuppressive therapy for scleroderma. The growing scleroderma drug pipeline, with new immunosuppressants and biosimilars meant to enhance efficacy and safety profiles, supports the segment's predominance even more.
Since the endothelin receptor antagonist (ERA) sector treats PAH, a life-threatening condition, it is expected to register the quickest CAGR. ERAs like Bosentan and Macitentan, as approved by the FDA, together with orphan medication designations, have sped their acceptance. Clinical studies sponsored by governments keep confirming their long-term advantages in lowering vascular remodeling and enhancing exercise tolerance for scleroderma patients. The fast expansion of this category is underlined by the increasing load of PAH globally and improved regulatory support for disease-modifying therapies. For scleroderma-associated PAH, for example, recent NIH-funded studies have investigated combination treatments including ERAs and biologics, underscoring the dynamic character of the scleroderma therapeutic market.
Regional Analysis:
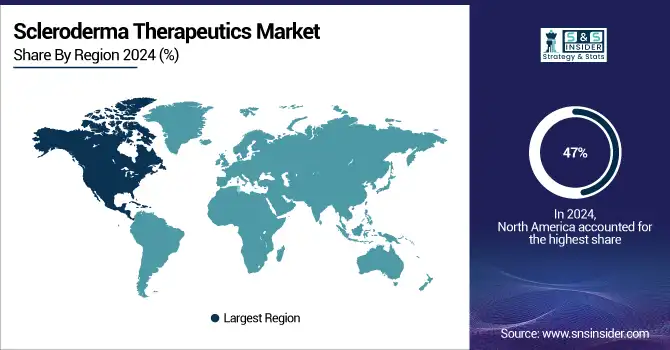
Dominating the global scleroderma therapeutics market in 2024 and accounting for over 47% of the market revenue share, North America. The great frequency of scleroderma, especially systemic sclerosis, in the United States and Canada, together with a well-established healthcare system supporting early diagnosis and sophisticated treatment access, helps to explain this dominance. Strong regulatory environment, including incentives like the Orphan Drug Act, which promotes pharmaceutical innovation and speeds the approval of new treatments, supports the U.S. Scleroderma Therapeutics industry. For instance, Aisa Pharmaceuticals's calcium channel blocker Profervia got FDA orphan drug designation for scleroderma treatment in September 2024, therefore underscoring the region's active involvement in improving the scleroderma treatment industry.
Get Customized Report as per Your Business Requirement - Enquiry Now
Driven by expanding illness awareness, better healthcare infrastructure, and more research and development funding, the Asia-Pacific area is the fastest-growing market for scleroderma treatments. Driven by government programs to improve autoimmune disease management, scleroderma diagnosis is rising in nations such as Japan, China, and India. Particularly, Japan has expedited the introduction of novel biologics for scleroderma and localized scleroderma drugs by streamlining orphan drug approvals. Together with rising patient numbers, the increasing pharmaceutical sector in Asia-Pacific is predicted to be a major contributor to the Scleroderma Therapeutics market expansion in this region.
Supported by government healthcare policies encouraging early diagnosis and access to improved treatments for systemic sclerosis and localized scleroderma, Europe holds a significant portion of the global market. National measures include funding for clinical research and patient support programs have been adopted by nations such Germany, the UK, and France to help to control autoimmune diseases. The European Medicines Agency (EMA) has also enabled orphan drug designations, therefore accelerating the approval of scleroderma disease-modifying treatments.
The Latin America, Middle East, and Africa (LAMEA) region held a significant share. Rising healthcare costs, bettering medical infrastructure, and government programs meant to raise awareness of and diagnosis of autoimmune illnesses drive development in these areas. Investing in rare illness frameworks, nations such as Brazil and South Africa are helping scleroderma sufferers financially and encouraging research partnerships. Though market penetration is already less than in developed areas, the LAMEA market is set for fast expansion as access to healthcare and innovative therapy use rise.
Scleroderma Therapeutics Market Key Players:
The key Scleroderma Therapeutics Companies are Bayer AG, Bristol-Myers Squibb Company, Boehringer Ingelheim International GmbH, F. Hoffman La-Roche Ltd., Chemomab, Emerald Health Pharmaceuticals, Cytori Therapeutics Inc., Corbus Pharmaceuticals Holdings, Inc., Calliditas Therapeutics AB, Sanofi., others
Recent Developments in the Scleroderma Therapeutics Market:
-
In May 2025, UCSF initiated clinical trials for Vixarelimab, targeting systemic sclerosis-associated interstitial lung disease, reflecting government-supported efforts to address lung complications in scleroderma.
-
In January 2025, European health agencies launched a collaborative initiative to enhance diagnostic precision and treatment access for systemic sclerosis patients, supported by government grants emphasizing patient-centered care and innovative biologics for scleroderma.
Scleroderma Therapeutics Market Report Scope:
| Report Attributes | Details |
|---|---|
| Market Size in 2024 | USD 2.55 Billion |
| Market Size by 2032 | USD 3.84 Billion |
| CAGR | CAGR of 5.28% From 2025 to 2032 |
| Base Year | 2024 |
| Forecast Period | 2025-2032 |
| Historical Data | 2021-2023 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Drug Class (Immunosuppressors, Calcium Channel Blockers, Endothelin Receptor Antagonists, Prostacyclin Analogues, Phosphodiesterase 5 inhibitors – PHA, Analgesics, Others) • By Indication (Systemic Scleroderma {Morphea, Linear}, Localized Scleroderma {Diffuse Systemic Sclerosis, Limited Cutaneous Systemic Sclerosis Syndrome}) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, Poland, Turkey, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Rest of Latin America) |
| Company Profiles | Bayer AG, Bristol-Myers Squibb Company, Boehringer Ingelheim International GmbH, F. Hoffman La-Roche Ltd., Chemomab, Emerald Health Pharmaceuticals, Cytori Therapeutics Inc., Corbus Pharmaceuticals Holdings, Inc., Calliditas Therapeutics AB, Sanofi., others |

