Transcatheter Mitral Valve Market Report Scope & Overview:
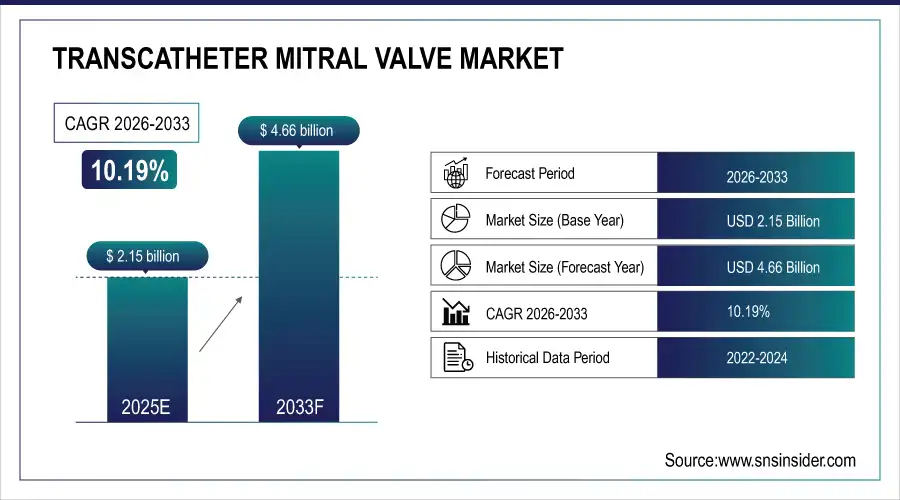
The global transcatheter mitral valve market size was valued at USD 2.15 billion in 2025E and is projected to reach USD 4.66 billion by 2033, growing at a CAGR of 10.19% during the forecast period 2026–2033.
The transcatheter mitral valve market is expanding rapidly, driven by the rising prevalence of mitral regurgitation affecting over 24 million people globally. The need for alternate approaches has led to increasing interest in less invasive transcatheter repair and replacement, as well as the development of improved, more functional bioprosthetic valve designs. Hospitals and specialist heart centers are the main treatment centers. Rising traction toward transseptal procedures, robust clinical trial initiatives and enhanced valve durability are driving market expansion across the globe.
Over 70% of transcatheter mitral valve procedures treat mitral regurgitation, with bioprosthetic valves making up nearly 80% of implants.
Market Size and Forecast:
-
Market Size in 2025: USD 2.15 Billion
-
Market Size by 2033: USD 4.66 Billion
-
CAGR: 10.19% from 2026 to 2033
-
Base Year: 2025
-
Forecast Period: 2026–2033
-
Historical Data: 2022–2024
To Get more information On Transcatheter Mitral Valve Market - Request Free Sample Report
Transcatheter Mitral Valve Market Trends:
-
Bioprosthetic transcatheter valves currently dominate are forecasted to account for more than 75% of the global implants by 2027.
-
Transseptal implantation is anticipated to witness a 35% growth during 2026-28 overtaking transapical access as the preferred modality.
-
There are more than 110 active clinical trials in 2025 involving next-generation mitral repair and replacement devices.
-
Specialty cardiac centers are projected to experience a 28% increase in procedure volumes by 2028 driven by centralization of advanced care.
-
Strong innovation, with adoption of new TMV products from almost 45% of top cardiovascular device manufacturers by 2029.
U.S. Transcatheter Mitral Valve Insights
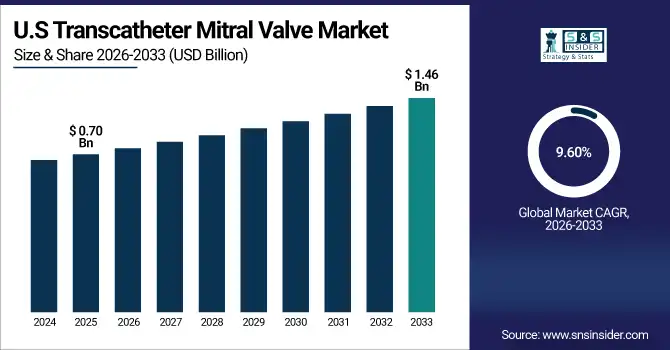
The U.S. leads the North American transcatheter mitral valve market, valued at USD 0.70 Billion in 2025E and projected to reach USD 1.46 Billion by 2033, growing at a CAGR of 9.60%. Over 1.2 million patients present with severe mitral regurgitation, making the demand for repair and replacement solutions a continuous one. Robust FDA clearance pipelines, attractive reimbursement landscapes and the existence of some of Medtech’s most innovative developers continue to underpin U.S. as a region-leading growth driver.
Transcatheter Mitral Valve Market Growth Drivers:
-
Aging Population and Rising Heart Failure Cases Drive Adoption of Transcatheter Mitral Valve Interventions Worldwide.
Demographic trends, as well as more patients who develop heart failure, are driving the demand for transcatheter mitral valve interventions. Nearly 24 million people worldwide are affected by mitral regurgitation, and prevalence is rising in patients over age 65. It Is the preferred substitute for the elderly, who are at increased risk for traditional open-heart surgery. Increasing use of transseptal procedures and long lasting bioprosthetic valves, also facilitating market development, is helped by the global tendency to promote advanced cardiovascular intervention systems in the healthcare industry.
In 2025, nearly 68% of transcatheter mitral valve procedures addressed mitral regurgitation, establishing it as the leading clinical indication driving adoption.
Transcatheter Mitral Valve Market Restraints:
-
High Cost and Procedural Complexity Restrain Market Growth, as Transcatheter Mitral Valve Devices Remain Less Accessible in Developing Regions.
The high price and procedural complexity still stifle the development of transcatheter mitral valve therapy market. The procedures are complex and dependent on advanced infrastructure and super specialists, which are not easily accessible. Indeed, less than 20% of cardiology centers are currently set up for transcatheter mitral valve interventions. Complexiti es in training and procedural risks make adoption difficult, especially in developing regions, inhibiting the growth to market despite significant minimally invasive cardiac solution demand.
Transcatheter Mitral Valve Market Opportunities:
-
Expanding Clinical Trials and Next-Generation Valve Innovations Create Opportunities, Advancing Global Adoption of Transcatheter Mitral Valve Therapies.
Increasing transcatheter mitral valve R&D is key growth opportunity. Over 95 trials are active in 2025 and are being designed to test next-generation surgical repair- and replacement-devices with more durable delivery systems. According to early trial data success rates of over 40% for advanced valve prototypes are reached, proving a high degree of innovation. As more regulatory approvals and evolving, minimally invasive, cardiology programs are realized the market potential is poised for global acceptance at an accelerated pace delivering better patient health.
Over 65% of interventional cardiologists favor next-generation transcatheter mitral valves for enhanced durability and procedural success, driving innovation-led adoption.
Transcatheter Mitral Valve Market Segmentation Analysis:
-
By Product Type, Transcatheter Mitral Valve Repair Devices is the largest segment with a market share of 54.88% in 2025E, while Transcatheter Mitral Valve Replacement Devices is the fastest-growing at a CAGR of 12.45%.
-
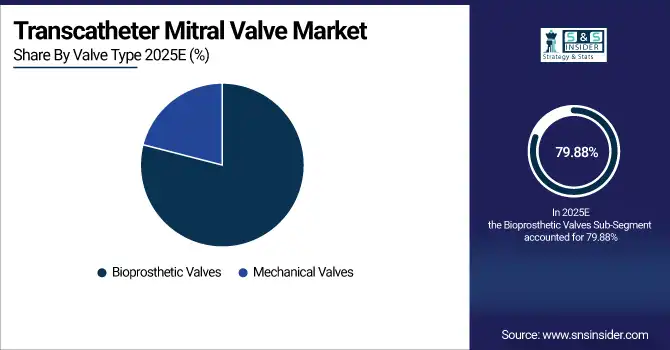
By Valve Type, Bioprosthetic Valves lead with 79.88% share in 2025E, and they also grow the fastest at a CAGR of 9.20%.
-
By Implantation Approach, Transseptal occupies the largest share of 48.84% in 2025E, and it is also the fastest-growing with a CAGR of 13.00%.
-
By Indication, Mitral Regurgitation is the dominant segment, accounting for 67.91% in 2025E, and also the fastest-growing with a CAGR of 11.20%.
-
By End User, Hospitals hold the majority share of 74.42% in 2025E, while Ambulatory Surgical Centers expand the fastest at a CAGR of 14.25%.
-
By Distribution Channel, Hospital Pharmacies dominate with a 67.44% share in 2025E, while Online Pharmacies witness the highest growth at a CAGR of 18.30%.
By Product Type, Repair Devices Dominate While Replacement Devices Grow Rapidly:
Transcatheter mitral valve repair devices also predominate, more than 68% TMV procedures in 2025 being for mitral regurgitation with repair as the preferred procedure. Repair has the advantage of lower procedural risk and quicker recovery so that it is more favorable for elderly patients. Replacement devices are proliferating fast, with more than 45 worldwide clinical trials underway of next-generation TMVR systems that could offer better valve durability and long-term results than alternatives like surgery.
By Valve Type, Bioprosthetic Valves Lead and Grow the Fastest:
Bioprosthesis Valves are the dominant market as over 70% TMV patients over 65 favor these valves considering less anticoagulation and safety results. There is clinical evidence of reduced complications compared with mechanical valves. They are also the fastest-growing, fueled by new tissue-engineering breakthroughs. More than 30 next-generation bioprosthetic designs are currently under development, enhancing valve durability and facilitating their use in repair and replacement procedures globally.
By Implantation Approach, Transseptal Dominates and Expands the Fastest:
Transseptal supremacy the issue at hand is that greater than 60% of TMV procedures performed in 2025 are via this less invasive alternative trans-septal entry the transapical technique with a much lower procedural risk. Demand by physicians is increasing as training programs and device availability grow. It is the fastest growing, and transseptal techniques account for more than 50% of active TMV clinical trials today which emphasizes that this will likely become the new standard route in mitral interventions.
By Indication, Mitral Regurgitation Dominates and Grows the Fastest:
The mitral regurgitation market is the largest patient population for TMV technologies, and nearly 24 million individuals around the globe suffer with it. Clinical trial data demonstrate substantial reductions in hospitalizations for heart failure patients having this condition who undergo transcatheter repair and replacement. It is also the fastest-expanding group and more than two-thirds of TMV device trials are dedicated to mitral regurgitation, underscoring a robust innovation pipeline focused on this common disease.
By End User, Hospitals Dominate While Ambulatory Centers Surge:
Hospitals will still lead as more than 80% of TMV procedures are performed in hospital by 2025, due to advanced imaging, hybrid cath labs and post-operative care. Hospitals continue to be the preferred treatment option with access to experienced cardiologists and dedicated facilities. Ambulatory surgical centers are booming, though as non-invasive interventions become safer: Outpatient TMV in particular is expected to surge by 40% between 2026 and 2028.
By Distribution Channel, Hospital Pharmacies Dominate While Online Platforms Accelerate:
Hospital pharmacies take precedence, with over 75% of TMV devices in 2025 being supplied via the hospital supply systems for scheduled procedures. Their scale in purchasing and hook to surgical scheduling are what make them sticky. However, online channels are increasingly becoming the rapid-rising distribution pipeline that it may have been hoped for digital purchases of cardiovascular devices to increase at more than 25% globally by 2027 reflecting a larger adoption of e-health and remote supply chains.
Transcatheter Mitral Valve Market Regional Analysis:
North America Transcatheter Mitral Valve Market Insights:
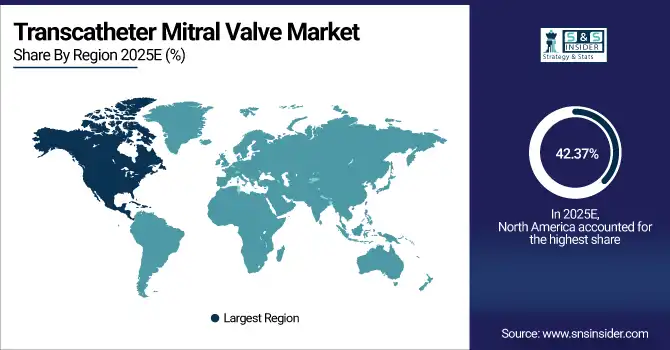
North America dominates the transcatheter mitral valve market with 42.37% share in 2025E, supported by advanced cardiovascular care and strong adoption of minimally invasive interventions. Only in the U.S., over 1.2 million patients have moderate-to-severe MR that generates a steady pool of patients for treatment with transcatheter repair and mitral replacement devices. FDA approvals are positive, reimbursement is robust and global Medtech leaders position North America as the early leader in transcatheter mitral valve innovation and clinical adoption.
Get Customized Report as per Your Business Requirement - Enquiry Now
U.S. Transcatheter Mitral Valve Market Insights:
The U.S. leads the transcatheter mitral valve market with more than 1,200 active clinical trials in 2025 focused on mitral repair and replacement. Almost 65% of high-volume cardiac centers have implemented novel transseptal methods with a positive impact on patient outcomes and procedural safety. Robust FDA approval pipelines and integration of AI-powered imaging technologies further highlight the U.S. as a center for transcatheter mitral valve innovation and broad-based clinical adoption.
Asia-Pacific Transcatheter Mitral Valve Market Insights:
The Asia-Pacific transcatheter mitral valve market is projected to grow at a CAGR of 11.71%, making it the fastest-growing region worldwide. More than 3 million people are diagnosed with severe left-sided mitral regurgitation each year in China and India, driving demand for less invasive solutions. Higher investments in cardiac care, expansion of catheterization labs, and medical tourism drive wider acceptance. Furthermore, over 70% of these top regional hospitals are adopting transcatheter valve therapies.
China Transcatheter Mitral Valve Market Insights:
China dominates the Asia-Pacific transcatheter mitral valve market, with over 1.5 million patients annually diagnosed with significant mitral regurgitation. The continued rise in spending, and strong government-backed cardiovascular programs are resulting in China being a regional hub for adoption and innovation with over 400 centers now active in performing TMV procedures.
Europe Transcatheter Mitral Valve Market Insights:
Europe also serves as one of the vital center for transcatheter mitral valve market with over 900,000 valve related intervention carried out annually in Germany, France, UK and Italy. In 2025, there were more than 2,300 active structural heart trials in the EU driving device innovation. With 92% of patients treated nearly fully covered under Public Healthcare, high access and substantial EU funding in cardiovascular research solidifies Europe’s helm seat as the leader in advanced TMV adoption.
Germany Transcatheter Mitral Valve Market Insights:
Germany dominates the European transcatheter mitral valve market, performing over 65,000 mitral valve interventions annually across more than 200 specialized cardiac centers. Diligent reimbursement policy, early transseptal approach adoption and a strong culture of clinical trial involvement systematically relegate Germany as the regional champion for innovation in addition to large volume TMV implementations.
Latin America Transcatheter Mitral Valve Market Insights:
Latin America’s transcatheter mitral valve market is expanding rapidly, supported by over 120,000 annual valve-related procedures across Brazil, Mexico, and Argentina. Brazil heads the list, with over 70 dedicated cardiac centers that perform TMV procedures. Increasing healthcare spending, better educations of interventional cardiologists and increasing use of minimally invasive procedures that expand the revenue growth in the region.
Middle East and Africa Transcatheter Mitral Valve Market Insights:
The Middle East and Africa transcatheter mitral valve market is expanding as specialized cardiac services become more accessible. It has been offered at more than 80 elite heart centers in UAE, Saudi Arabia and South Africa since 2025. Robust government spending in cardiovascular technology and growing medical tourism are driving adoption at a faster pace, setting the stage for long-term sustained growth.
Transcatheter Mitral Valve Market Competitive Landscape:
Edwards Lifesciences leads the transcatheter mitral valve market with its PASCAL and Cardio band systems, adopted in more than 35,000 patients worldwide by 2025. The company operates more than 60 active global clinical trials on TMV repair and replacement providing robust evidence for outcomes. The company's robust presence in over 120 countries and increasing investment in R&D position Edwards as the world leader in patient-focused innovations for structural heart disease, as well as critical care and surgical monitoring.
-
In March 2025, Edwards Lifesciences received CE Mark approval for its SAPIEN M3 system, the first transfemoral transcatheter mitral valve replacement therapy for patients unsuitable for surgery or repair.
Medtronic is a key player in the replacement segment, advancing with its Intrepid TMVR system, tested in over 2,500 patients through global trials. The firm functions in 140 countries, using its distribution and regulatory know-how to grow penetration. Medtronic’s pipeline of next-generation technologies, such as new transseptal delivery systems, and their dedication to patient outcomes set them apart as a dominant influencer in the global TMV replacement market.
-
In June 2025, Medtronic advanced its APOLLO trial evaluating the Intrepid TMVR device, enrolling over 2,500 patients with severe symptomatic mitral regurgitation.
Abbott dominates the repair segment with its MitraClip device, implanted in over 150,000 patients globally by 2025, making it the most widely used TMV repair solution. The company leverages FDA and CE approvals, broad reimbursement across 18 countries, and more than 50 active TMV clinical studies. Abbott’s portfolio, physician education initiatives and international supply chains further entrench its dominance in North American, European and developing Asia-Pacific markets.
-
In September 2025, Abbott gained FDA approval for its Tendyne TMVR system, offering a breakthrough replacement option for patients with severe mitral annular calcification.
Transcatheter Mitral Valve Market Key Players:
Some of the Transcatheter Mitral Valve Market Companies are:
-
Edwards Lifesciences Corporation
-
Medtronic plc
-
Abbott Laboratories
-
Boston Scientific Corporation
-
LivaNova PLC
-
Neovasc Inc.
-
JenaValve Technology, Inc.
-
Micro Interventional Devices, Inc.
-
Cardiac Dimensions, Inc.
-
HighLife SAS
-
Valtech Cardio Ltd.
-
Tendyne Holdings, Inc.
-
Mitralign, Inc.
-
4C Medical Technologies, Inc.
-
Cephea Valve Technologies, Inc.
-
MValve Technologies Ltd.
-
Transcatheter Technologies GmbH
-
Ancora Heart, Inc.
-
Navigate Cardiac Structures, Inc.
-
CryoLife, Inc.
| Report Attributes | Details |
|---|---|
| Market Size in 2025 | USD 2.15 Billion |
| Market Size by 2033 | USD 4.66 Billion |
| CAGR | CAGR of 10.19% From 2026 to 2033 |
| Base Year | 2025 |
| Forecast Period | 2026-2033 |
| Historical Data | 2022-2024 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product Type (Transcatheter Mitral Valve Repair Devices, Transcatheter Mitral Valve Replacement Devices, Others) • By Valve Type (Mechanical Valves, Bioprosthetic Valves) • By Implantation Approach (Transapical, Transseptal, Transfemoral, Others) • By Indication (Mitral Regurgitation, Mitral Stenosis, Others) • By End User (Hospitals, Ambulatory Surgical Centers, Specialty Cardiac Centers) • By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies) |
| Regional Analysis/Coverage | North America (US, Canada), Europe (Germany, UK, France, Italy, Spain, Russia, Poland, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Australia, ASEAN Countries, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Mexico, Colombia, Rest of Latin America). |
| Company Profiles | Edwards Lifesciences Corporation, Medtronic plc, Abbott Laboratories, Boston Scientific Corporation, LivaNova PLC, Neovasc Inc., JenaValve Technology, Inc., Micro Interventional Devices, Inc., Cardiac Dimensions, Inc., HighLife SAS, Valtech Cardio Ltd., Tendyne Holdings, Inc., Mitralign, Inc., 4C Medical Technologies, Inc., Cephea Valve Technologies, Inc., MValve Technologies Ltd., Transcatheter Technologies GmbH, Ancora Heart, Inc., Navigate Cardiac Structures, Inc., CryoLife, Inc. |

