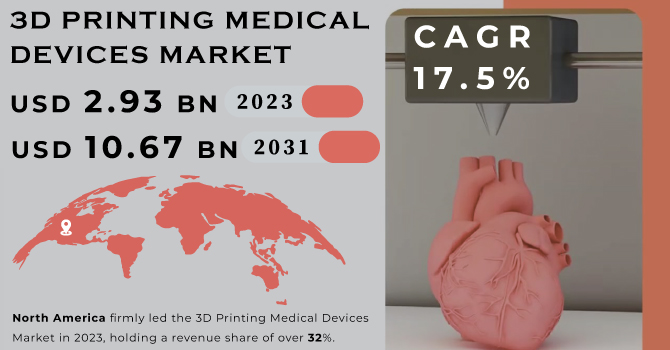
The 3D Printing Medical Devices Market Size was valued at USD 2.93 billion in 2023 and is expected to reach USD 10.67 billion by 2031 and grow at a CAGR of 17.5% over the forecast period 2024-2031.
3D printing significantly reduces the lead time for producing medical devices, accelerating the path from concept to market and allowing fast response to emerging medical needs. 3D printing enables the creation of intricate geometries not feasible with traditional manufacturing. This opens up possibilities for devices with improved biocompatibility, tissue integration, and more natural functionality. 3D Systems offers a wide range of 3D printing technologies and materials targeted at medical applications. Governments worldwide are investing in 3D printing research and development. Regulatory agencies are adapting guidelines to facilitate the approval of 3D-printed medical devices. For example, the US FDA has issued guidance documents specifically for 3D-printed medical devices and encourages collaboration in research.
Get more information on 3D Printing Medical Devices Market - Request Sample Report
3D printing allows for unprecedented customization of medical devices. This includes
Prosthetics and Implants can be tailored precisely to a patient's anatomy, improving fit, comfort, and functionality.
Surgical Guides and Models that allow for more accurate surgical planning, leading to better outcomes.
Patient-Specific Drug Delivery Devices which can be optimized for an individual's needs.
MARKET DYNAMICS
Drivers
Rapid prototyping capabilities enable quicker development and iteration of medical devices
Reduced costs in manufacturing and assembly compared to traditional methods.
Increasing acceptance of 3D-printed medical devices by regulatory bodies like the FDA facilitates market growth.
Growing demand for implants, prosthetics, and surgical instruments due to an aging population and increasing healthcare needs.
Advancements in technology make 3D printing more accessible to smaller healthcare facilities and developing regions.
Restraint
Concerns over IP protection in the context of easily replicable 3D-printed devices
Limited availability of biocompatible materials suitable for medical device manufacturing.
Lack of expertise among healthcare professionals in utilizing and understanding 3D printing technology for medical applications.
Opportunities
The increasing use of CAD/CAM technology (computer-aided design/manufacturing) alongside affordable desktop printers
Potential for personalized medical devices fabricated rapidly at the point of care, tailored to individual patient needs.
Increasing demand for customized implants and medical devices tailored to individual patient needs.
Potential expansion of 3D printing beyond devices to include tissue engineering and pharmaceuticals.
Challenges
Stringent regulatory processes, especially in the US, can take years to approve new 3D-printed devices
Lack of standardized processes and guidelines for 3D printing in the medical field, leading to variability in quality and outcomes
Impact of Russia- Ukraine War
Ukraine was a manufacturer of some 3D printers, particularly the IDEX model by AddWise. The conflict has halted production and made it difficult to transport these printers, impacting global availability. Additionally, raw materials and components for 3D printers may be sourced from regions affected by the war, causing shortages and price increases. The overall economic uncertainty due to the war discourages investment in new technologies and research and development (R&D). This can slow down the advancement of 3D printing technologies specifically for medical devices. Many leading 3D printing companies have halted business with Russia due to sanctions, further limiting market reach. The war's impact on energy prices and inflation can have a ripple effect, affecting the affordability of 3D printers and materials for medical device production worldwide.
KEY MARKET SEGMENTS:
By Technology
Laser Beam Melting
Electron Beam Melting
Photopolymerization
Droplet Deposition
Three-dimensional Printing /Binder Jetting
Other Technologies
The laser beam melting (LBM) segment had the biggest market share. The growing use of this technology in the dental industry and for manufacturing implants for minimally invasive surgery is responsible for a huge proportion of this market.
By Component
Materials
Equipment
Services & Software
The market for 3D printing medical devices is divided into three categories: equipment, materials, and software and services. The 3D Printing Medical Devices market was dominated by software and services. The cost-effectiveness, utility, consistency, and precision of medical device 3D printing services are likely to drive this segment's expansion over the forecast period.
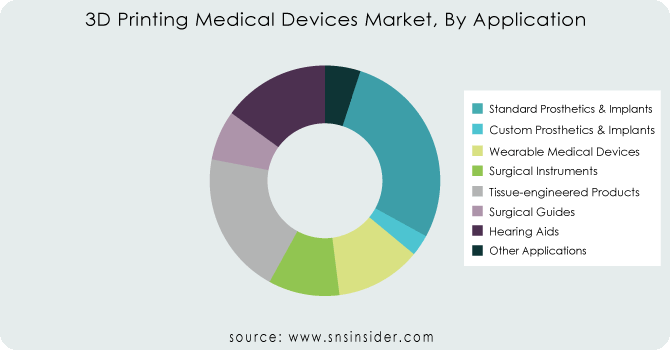
By Application
Standard Prosthetics & Implants
Custom Prosthetics & Implants
Surgical Instruments
Tissue-engineered Products
Surgical Guides
Other Applications
In 2023, the custom prosthetics and implants category held a higher part of the market. 3D printing is widely used to create highly customizable prosthetics and implants from a wide range of materials, including biological materials (such as skin and bones), plastics, ceramics, and metals. 3D printing of custom implants is drawing many medical device businesses and new investors to purchase 3D printers. This market segment is predicted to grow rapidly in the future years.
Need any customization research on 3D Printing Medical Devices Market - Enquiry Now
By End User
Hospitals & Surgical Centers
Academic Institutions & Research Laboratories
Dental & Orthopedic Clinics
Clinical Research Organizations
Pharma-Biotech & Medical Device Companies
In 2023, the hospital and surgical center category accounted for the greatest proportion of the market. The growth of existing 3D printing laboratories, the increasing affordability of 3D printing services, and hospitals' quick embrace of sophisticated technologies are all contributing to this segment's big share.
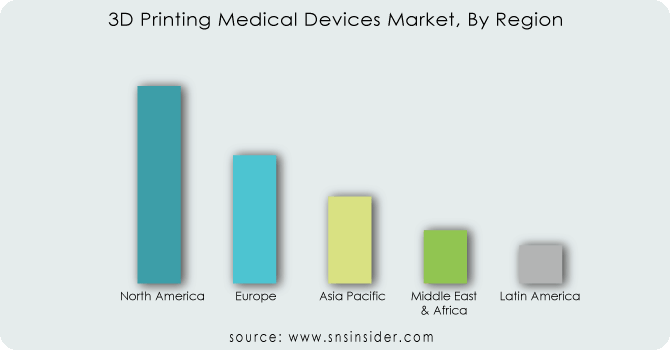
REGIONAL ANALYSIS
North America firmly led the 3D Printing Medical Devices Market in 2023, holding a revenue share of over 32%. The region boasts a robust healthcare system and renowned research institutions like the Mayo Clinic. These centers readily embrace emerging technologies like 3D printing, using them for surgical planning and patient-specific models to enhance patient outcomes. Governments in North America, especially the US, proactively support the advancement of 3D printing. The FDA's tailored guidance for 3D-printed medical devices streamlines the approval process and promotes innovation.
REGIONAL COVERAGE:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
KEY PLAYERS:
3D Systems Corporations, Cyfuse Medical K.K., Stratasys Ltd., EOS GmbH, 3T RPD Ltd., Prodways Group, Oxford Performance Materials, Inc., SLM Solutions Group AG, Arcam AB, EnvisionTEC, Organovo Holdings, Inc., Bio3D Technologies, Renishaw plc, Materialise NV, Laser GmbH
Cyfuse Medical K.K-Company Financial Analysis
Recent Development
In April 2024, 3D Systems made a significant announcement regarding their latest innovation in the medical field. The Food and Drug Administration (FDA) has granted 510(k) clearance for their groundbreaking 3D-printed cranial implant solution, known as the VSP® PEEK Cranial Implant. This product includes a comprehensive FDA-approved workflow that incorporates segmentation and 3D modeling software, the state-of-the-art 3D Systems EXT 220 MED 3D printer, Evonik VESTAKEEP® i4 3DF PEEK material, and a meticulously defined production process.
In November 2023, Stratasys Ltd., a renowned leader in polymer 3D printing and additive manufacturing solutions, announced a groundbreaking partnership with Siemens Healthineers. This collaboration aims to conduct a groundbreaking research project focused on developing advanced solutions for medical imaging phantoms used in computed tomography (CT) imaging.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 2.93 Billion |
| Market Size by 2031 | US$ 10.67 Billion |
| CAGR | CAGR of 17.5% From 2023 to 2030 |
| Base Year | 2023 |
| Forecast Period | 2024-2031 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Technology (Laser Beam Melting, Electron Beam Melting, Photopolymerization, Droplet Deposition, Three-dimensional Printing /Binder Jetting, Other Technologies) • By Component (Materials, Equipment, Services & Software) • By Application (Standard Prosthetics & Implants, Custom Prosthetics & Implants, Wearable Medical Devices, Surgical Instruments, Tissue-engineered Products, Surgical Guides, Hearing Aids, and Other Applications) • By End User (Hospitals & Surgical Centers, Academic Institutions & Research Laboratories, Dental & Orthopedic Clinics, Clinical Research Organizations, Pharma-Biotech & Medical Device Companies) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | 3D Systems Corporations, Cyfuse Medical K.K., Stratasys Ltd., EOS GmbH, 3T RPD Ltd., Prodways Group, Oxford Performance Materials, Inc., SLM Solutions Group AG, Arcam AB, EnvisionTEC, Organovo Holdings, Inc., Bio3D Technologies, Renishaw plc, Materialise NV, Laser GmbH |
| DRIVERS | • Rapid prototyping capabilities enable quicker development and iteration of medical devices • Reduced costs in manufacturing and assembly compared to traditional methods. • Increasing acceptance of 3D printed medical devices by regulatory bodies like the FDA, facilitating market growth. • Growing demand for implants, prosthetics, and surgical instruments due to an aging population and increasing healthcare needs. • Advancements in technology make 3D printing more accessible to smaller healthcare facilities and developing regions. |
| RESTRAINTS | • Concerns over IP protection in the context of easily replicable 3D-printed devices • Limited availability of biocompatible materials suitable for medical device manufacturing. • Lack of expertise among healthcare professionals in utilizing and understanding 3D printing technology for medical applications. |
Ans:- The 3D Printing Medical Devices Market Size was valued at USD 2.93 billion in 2023.
3D Systems Corporations, Cy fuse Medical K.K., Stratasys Ltd., EOS GmbH Concept, 3T RPD Ltd., Prod ways Group, Oxford Performance Materials, Inc., SLM Solutions Group AG, Arcam AB are the key players of the 3D Printing Medical Devices Market.
Ans:- The 3D Printing Medical Devices Market is growing at a CAGR of 17.5% over the forecast period 2024-2031.
Increasing the public-private partnership's funding, Ability to conduct research and development, are all propelling the 3D Printing Medical Devices market forward.
Due to a lack of specialized training, and Skilled labor shortage are the main restraints of the Medical Nonwoven Disposable market.
TABLE OF CONTENTS
1. Introduction
1.1 Market Definition
1.2 Scope
1.3 Research Assumptions
2. Industry Flowchart
3. Research Methodology
4. Market Dynamics
4.1 Drivers
4.2 Restraints
4.3 Opportunities
4.4 Challenges
5. Impact Analysis
5.1 Impact of Russia-Ukraine Crisis
5.2 Impact of Economic Slowdown on Major Countries
6. Value Chain Analysis
7. Porter’s 5 Forces Model
8. Pest Analysis
9. 3D Printing Medical Devices Market Segmentation, By Technology
9.1 Introduction
9.2 Trend Analysis
9.3 Laser Beam Melting
9.4 Electron Beam Melting
9.5 Photopolymerization
9.6 Droplet Deposition
9.7 Three-dimensional Printing /Binder Jetting
9.8 Other Technologies
10. 3D Printing Medical Devices Market Segmentation, By Component
10.1 Introduction
10.2 Trend Analysis
10.3 Materials
10.4 Equipment
10.5 Services & Software
11. 3D Printing Medical Devices Market Segmentation, By Application
11.1 Introduction
11.2 Trend Analysis
11.3 Standard Prosthetics & Implants
11.4 Custom Prosthetics & Implants
11.5 Wearable Medical Devices
11.6 Surgical Instruments
11.7 Tissue-engineered Products
11.8 Surgical Guides
11.9 Hearing Aids
11.10 Other Applications
12. 3D Printing Medical Devices Market Segmentation, By End User
12.1 Introduction
12.2 Trend Analysis
12. 3Hospitals & Surgical Centers
12.4 Academic Institutions & Research Laboratories
12.5 Dental & Orthopedic Clinics
12.6 Clinical Research Organizations
12.7 Pharma-Biotech & Medical Device Companies
13. Regional Analysis
13.1 Introduction
13.2 North America
13.2.1 Trend Analysis
13.2.2 North America 3D Printing Medical Devices Market by Country
13.2.3 North America 3D Printing Medical Devices Market By Technology
13.2.4 North America 3D Printing Medical Devices Market By Component
13.2.5 North America 3D Printing Medical Devices Market By Application
13.2.6 North America 3D Printing Medical Devices Market By End User
13.2.7 USA
13.2.7.1 USA 3D Printing Medical Devices Market By Technology
13.2.7.2 USA 3D Printing Medical Devices Market By Component
13.2.7.3 USA 3D Printing Medical Devices Market By Application
13.2.7.4 USA 3D Printing Medical Devices Market By End User
13.2.8 Canada
13.2.8.1 Canada 3D Printing Medical Devices Market By Technology
13.2.8.2 Canada 3D Printing Medical Devices Market By Component
13.2.8.3 Canada 3D Printing Medical Devices Market By Application
13.2.8.4 Canada 3D Printing Medical Devices Market By End User
13.2.9 Mexico
13.2.9.1 Mexico 3D Printing Medical Devices Market By Technology
13.2.9.2 Mexico 3D Printing Medical Devices Market By Component
13.2.9.3 Mexico 3D Printing Medical Devices Market By Application
13.2.9.4 Mexico 3D Printing Medical Devices Market By End User
13.3 Europe
13.3.1 Trend Analysis
13.3.2 Eastern Europe
13.3.2.1 Eastern Europe 3D Printing Medical Devices Market by Country
13.3.2.2 Eastern Europe 3D Printing Medical Devices Market By Technology
13.3.2.3 Eastern Europe 3D Printing Medical Devices Market By Component
13.3.2.4 Eastern Europe 3D Printing Medical Devices Market By Application
13.3.2.5 Eastern Europe 3D Printing Medical Devices Market By End User
13.3.2.6 Poland
13.3.2.6.1 Poland 3D Printing Medical Devices Market By Technology
13.3.2.6.2 Poland 3D Printing Medical Devices Market By Component
13.3.2.6.3 Poland 3D Printing Medical Devices Market By Application
13.3.2.6.4 Poland 3D Printing Medical Devices Market By End User
13.3.2.7 Romania
13.3.2.7.1 Romania 3D Printing Medical Devices Market By Technology
13.3.2.7.2 Romania 3D Printing Medical Devices Market By Component
13.3.2.7.3 Romania 3D Printing Medical Devices Market By Application
13.3.2.7.4 Romania 3D Printing Medical Devices Market By End User
13.3.2.8 Hungary
13.3.2.8.1 Hungary 3D Printing Medical Devices Market By Technology
13.3.2.8.2 Hungary 3D Printing Medical Devices Market By Component
13.3.2.8.3 Hungary 3D Printing Medical Devices Market By Application
13.3.2.8.4 Hungary 3D Printing Medical Devices Market By End User
13.3.2.9 Turkey
13.3.2.9.1 Turkey 3D Printing Medical Devices Market By Technology
13.3.2.9.2 Turkey 3D Printing Medical Devices Market By Component
13.3.2.9.3 Turkey 3D Printing Medical Devices Market By Application
13.3.2.9.4 Turkey 3D Printing Medical Devices Market By End User
13.3.2.10 Rest of Eastern Europe
13.3.2.10.1 Rest of Eastern Europe 3D Printing Medical Devices Market By Technology
13.3.2.10.2 Rest of Eastern Europe 3D Printing Medical Devices Market By Component
13.3.2.10.3 Rest of Eastern Europe 3D Printing Medical Devices Market By Application
13.3.2.10.4 Rest of Eastern Europe 3D Printing Medical Devices Market By End User
13.3.3 Western Europe
13.3.3.1 Western Europe 3D Printing Medical Devices Market by Country
13.3.3.2 Western Europe 3D Printing Medical Devices Market By Technology
13.3.3.3 Western Europe 3D Printing Medical Devices Market By Component
13.3.3.4 Western Europe 3D Printing Medical Devices Market By Application
13.3.3.5 Western Europe 3D Printing Medical Devices Market By End User
13.3.3.6 Germany
13.3.3.6.1 Germany 3D Printing Medical Devices Market By Technology
13.3.3.6.2 Germany 3D Printing Medical Devices Market By Component
13.3.3.6.3 Germany 3D Printing Medical Devices Market By Application
13.3.3.6.4 Germany 3D Printing Medical Devices Market By End User
13.3.3.7 France
13.3.3.7.1 France 3D Printing Medical Devices Market By Technology
13.3.3.7.2 France 3D Printing Medical Devices Market By Component
13.3.3.7.3 France 3D Printing Medical Devices Market By Application
13.3.3.7.4 France 3D Printing Medical Devices Market By End User
13.3.3.8 UK
13.3.3.8.1 UK 3D Printing Medical Devices Market By Technology
13.3.3.8.2 UK 3D Printing Medical Devices Market By Component
13.3.3.8.3 UK 3D Printing Medical Devices Market By Application
13.3.3.8.4 UK 3D Printing Medical Devices Market By End User
13.3.3.9 Italy
13.3.3.9.1 Italy 3D Printing Medical Devices Market By Technology
13.3.3.9.2 Italy 3D Printing Medical Devices Market By Component
13.3.3.9.3 Italy 3D Printing Medical Devices Market By Application
13.3.3.9.4 Italy 3D Printing Medical Devices Market By End User
13.3.3.10 Spain
13.3.3.10.1 Spain 3D Printing Medical Devices Market By Technology
13.3.3.10.2 Spain 3D Printing Medical Devices Market By Component
13.3.3.10.3 Spain 3D Printing Medical Devices Market By Application
13.3.3.10.4 Spain 3D Printing Medical Devices Market By End User
13.3.3.11 Netherlands
13.3.3.11.1 Netherlands 3D Printing Medical Devices Market By Technology
13.3.3.11.2 Netherlands 3D Printing Medical Devices Market By Component
13.3.3.11.3 Netherlands 3D Printing Medical Devices Market By Application
13.3.3.11.4 Netherlands 3D Printing Medical Devices Market By End User
13.3.3.12 Switzerland
13.3.3.12.1 Switzerland 3D Printing Medical Devices Market By Technology
13.3.3.12.2 Switzerland 3D Printing Medical Devices Market By Component
13.3.3.12.3 Switzerland 3D Printing Medical Devices Market By Application
13.3.3.12.4 Switzerland 3D Printing Medical Devices Market By End User
13.3.3.13 Austria
13.3.3.13.1 Austria 3D Printing Medical Devices Market By Technology
13.3.3.13.2 Austria 3D Printing Medical Devices Market By Component
13.3.3.13.3 Austria 3D Printing Medical Devices Market By Application
13.3.3.13.4 Austria 3D Printing Medical Devices Market By End User
13.3.3.14 Rest of Western Europe
13.3.3.14.1 Rest of Western Europe 3D Printing Medical Devices Market By Technology
13.3.3.14.2 Rest of Western Europe 3D Printing Medical Devices Market By Component
13.3.3.14.3 Rest of Western Europe 3D Printing Medical Devices Market By Application
13.3.3.14.4 Rest of Western Europe 3D Printing Medical Devices Market By End User
13.4 Asia-Pacific
13.4.1 Trend Analysis
13.4.2 Asia-Pacific 3D Printing Medical Devices Market by Country
13.4.3 Asia-Pacific 3D Printing Medical Devices Market By Technology
13.4.4 Asia-Pacific 3D Printing Medical Devices Market By Component
13.4.5 Asia-Pacific 3D Printing Medical Devices Market By Application
13.4.6 Asia-Pacific 3D Printing Medical Devices Market By End User
13.4.7 China
13.4.7.1 China 3D Printing Medical Devices Market By Technology
13.4.7.2 China 3D Printing Medical Devices Market By Component
13.4.7.3 China 3D Printing Medical Devices Market By Application
13.4.7.4 China 3D Printing Medical Devices Market By End User
13.4.8 India
13.4.8.1 India 3D Printing Medical Devices Market By Technology
13.4.8.2 India 3D Printing Medical Devices Market By Component
13.4.8.3 India 3D Printing Medical Devices Market By Application
13.4.8.4 India 3D Printing Medical Devices Market By End User
13.4.9 Japan
13.4.9.1 Japan 3D Printing Medical Devices Market By Technology
13.4.9.2 Japan 3D Printing Medical Devices Market By Component
13.4.9.3 Japan 3D Printing Medical Devices Market By Application
13.4.9.4 Japan 3D Printing Medical Devices Market By End User
13.4.10 South Korea
13.4.10.1 South Korea 3D Printing Medical Devices Market By Technology
13.4.10.2 South Korea 3D Printing Medical Devices Market By Component
13.4.10.3 South Korea 3D Printing Medical Devices Market By Application
13.4.10.4 South Korea 3D Printing Medical Devices Market By End User
13.4.11 Vietnam
13.4.11.1 Vietnam 3D Printing Medical Devices Market By Technology
13.4.11.2 Vietnam 3D Printing Medical Devices Market By Component
13.4.11.3 Vietnam 3D Printing Medical Devices Market By Application
13.4.11.4 Vietnam 3D Printing Medical Devices Market By End User
13.4.12 Singapore
13.4.12.1 Singapore 3D Printing Medical Devices Market By Technology
13.4.12.2 Singapore 3D Printing Medical Devices Market By Component
13.4.12.3 Singapore 3D Printing Medical Devices Market By Application
13.4.12.4 Singapore 3D Printing Medical Devices Market By End User
13.4.13 Australia
13.4.13.1 Australia 3D Printing Medical Devices Market By Technology
13.4.13.2 Australia 3D Printing Medical Devices Market By Component
13.4.13.3 Australia 3D Printing Medical Devices Market By Application
13.4.13.4 Australia 3D Printing Medical Devices Market By End User
13.4.14 Rest of Asia-Pacific
13.4.14.1 Rest of Asia-Pacific 3D Printing Medical Devices Market By Technology
13.4.14.2 Rest of Asia-Pacific 3D Printing Medical Devices Market By Component
13.4.14.3 Rest of Asia-Pacific 3D Printing Medical Devices Market By Application
13.4.14.4 Rest of Asia-Pacific 3D Printing Medical Devices Market By End User
13.5 Middle East & Africa
13.5.1 Trend Analysis
13.5.2 Middle East
13.5.2.1 Middle East 3D Printing Medical Devices Market by Country
13.5.2.2 Middle East 3D Printing Medical Devices Market By Technology
13.5.2.3 Middle East 3D Printing Medical Devices Market By Component
13.5.2.4 Middle East 3D Printing Medical Devices Market By Application
13.5.2.5 Middle East 3D Printing Medical Devices Market By End User
13.5.2.6 UAE
13.5.2.6.1 UAE 3D Printing Medical Devices Market By Technology
13.5.2.6.2 UAE 3D Printing Medical Devices Market By Component
13.5.2.6.3 UAE 3D Printing Medical Devices Market By Application
13.5.2.6.4 UAE 3D Printing Medical Devices Market By End User
13.5.2.7 Egypt
13.5.2.7.1 Egypt 3D Printing Medical Devices Market By Technology
13.5.2.7.2 Egypt 3D Printing Medical Devices Market By Component
13.5.2.7.3 Egypt 3D Printing Medical Devices Market By Application
13.5.2.7.4 Egypt 3D Printing Medical Devices Market By End User
13.5.2.8 Saudi Arabia
13.5.2.8.1 Saudi Arabia 3D Printing Medical Devices Market By Technology
13.5.2.8.2 Saudi Arabia 3D Printing Medical Devices Market By Component
13.5.2.8.3 Saudi Arabia 3D Printing Medical Devices Market By Application
13.5.2.8.4 Saudi Arabia 3D Printing Medical Devices Market By End User
13.5.2.9 Qatar
13.5.2.9.1 Qatar 3D Printing Medical Devices Market By Technology
13.5.2.9.2 Qatar 3D Printing Medical Devices Market By Component
13.5.2.9.3 Qatar 3D Printing Medical Devices Market By Application
13.5.2.9.4 Qatar 3D Printing Medical Devices Market By End User
13.5.2.10 Rest of Middle East
13.5.2.10.1 Rest of Middle East 3D Printing Medical Devices Market By Technology
13.5.2.10.2 Rest of Middle East 3D Printing Medical Devices Market By Component
13.5.2.10.3 Rest of Middle East 3D Printing Medical Devices Market By Application
13.5.2.10.4 Rest of Middle East 3D Printing Medical Devices Market By End User
13.5.3 Africa
13.5.3.1 Africa 3D Printing Medical Devices Market by Country
13.5.3.2 Africa 3D Printing Medical Devices Market By Technology
13.5.3.3 Africa 3D Printing Medical Devices Market By Component
13.5.3.4 Africa 3D Printing Medical Devices Market By Application
13.5.3.5 Africa 3D Printing Medical Devices Market By End User
13.5.3.6 Nigeria
13.5.3.6.1 Nigeria 3D Printing Medical Devices Market By Technology
13.5.3.6.2 Nigeria 3D Printing Medical Devices Market By Component
13.5.3.6.3 Nigeria 3D Printing Medical Devices Market By Application
13.5.3.6.4 Nigeria 3D Printing Medical Devices Market By End User
13.5.3.7 South Africa
13.5.3.7.1 South Africa 3D Printing Medical Devices Market By Technology
13.5.3.7.2 South Africa 3D Printing Medical Devices Market By Component
13.5.3.7.3 South Africa 3D Printing Medical Devices Market By Application
13.5.3.7.4 South Africa 3D Printing Medical Devices Market By End User
13.5.3.8 Rest of Africa
13.5.3.8.1 Rest of Africa 3D Printing Medical Devices Market By Technology
13.5.3.8.2 Rest of Africa 3D Printing Medical Devices Market By Component
13.5.3.8.3 Rest of Africa 3D Printing Medical Devices Market By Application
13.5.3.8.4 Rest of Africa 3D Printing Medical Devices Market By End User
13.6 Latin America
13.6.1 Trend Analysis
13.6.2 Latin America 3D Printing Medical Devices Market by Country
13.6.3 Latin America 3D Printing Medical Devices Market By Technology
13.6.4 Latin America 3D Printing Medical Devices Market By Component
13.6.5 Latin America 3D Printing Medical Devices Market By Application
13.6.6 Latin America 3D Printing Medical Devices Market By End User
13.6.7 Brazil
13.6.7.1 Brazil 3D Printing Medical Devices Market By Technology
13.6.7.2 Brazil 3D Printing Medical Devices Market By Component
13.6.7.3 Brazil 3D Printing Medical Devices Market By Application
13.6.7.4 Brazil 3D Printing Medical Devices Market By End User
13.6.8 Argentina
13.6.8.1 Argentina 3D Printing Medical Devices Market By Technology
13.6.8.2 Argentina 3D Printing Medical Devices Market By Component
13.6.8.3 Argentina 3D Printing Medical Devices Market By Application
13.6.8.4 Argentina 3D Printing Medical Devices Market By End User
13.6.9 Colombia
13.6.9.1 Colombia 3D Printing Medical Devices Market By Technology
13.6.9.2 Colombia 3D Printing Medical Devices Market By Component
13.6.9.3 Colombia 3D Printing Medical Devices Market By Application
13.6.9.4 Colombia 3D Printing Medical Devices Market By End User
13.6.10 Rest of Latin America
13.6.10.1 Rest of Latin America 3D Printing Medical Devices Market By Technology
13.6.10.2 Rest of Latin America 3D Printing Medical Devices Market By Component
13.6.10.3 Rest of Latin America 3D Printing Medical Devices Market By Application
13.6.10.4 Rest of Latin America 3D Printing Medical Devices Market By End User
14. Company Profiles
14.1 3D Systems Corporations
14.1.1 Company Overview
14.1.2 Financial
14.1.3 Products/ Services Offered
14.1.4 SWOT Analysis
14.1.5 The SNS View
14.2 Cyfuse Medical K.K.
14.2.1 Company Overview
14.2.2 Financial
14.2.3 Products/ Services Offered
14.2.4 SWOT Analysis
14.2.5 The SNS View
14.3 Stratasys Ltd.
14.3.1 Company Overview
14.3.2 Financial
14.3.3 Products/ Services Offered
14.3.4 SWOT Analysis
14.3.5 The SNS View
14.4 EOS GmbH
14.4.1 Company Overview
14.4.2 Financial
14.4.3 Products/ Services Offered
14.4.4 SWOT Analysis
14.4.5 The SNS View
14.5 3T RPD Ltd.
14.5.1 Company Overview
14.5.2 Financial
14.5.3 Products/ Services Offered
14.5.4 SWOT Analysis
14.5.5 The SNS View
14.6 Prodways Group
14.6.1 Company Overview
14.6.2 Financial
14.6.3 Products/ Services Offered
14.6.4 SWOT Analysis
14.6.5 The SNS View
14.7 Oxford Performance Materials, Inc.
14.7.1 Company Overview
14.7.2 Financial
14.7.3 Products/ Services Offered
14.7.4 SWOT Analysis
14.7.5 The SNS View
14.8 SLM Solutions Group AG
14.8.1 Company Overview
14.8.2 Financial
14.8.3 Products/ Services Offered
14.8.4 SWOT Analysis
14.8.5 The SNS View
14.9 Arcam AB
14.9.1 Company Overview
14.9.2 Financial
14.9.3 Products/ Services Offered
14.9.4 SWOT Analysis
14.9.5 The SNS View
14.10 EnvisionTEC
14.10.1 Company Overview
14.10.2 Financial
14.10.3 Products/ Services Offered
14.10.4 SWOT Analysis
14.10.5 The SNS View
14.11 Organovo Holdings, Inc.
14.11.1 Company Overview
14.11.2 Financial
14.11.3 Products/ Services Offered
14.11.4 SWOT Analysis
14.11.5 The SNS View
14.12 Bio3D Technologies
14.12.1 Company Overview
14.12.2 Financial
14.12.3 Products/ Services Offered
14.12.4 SWOT Analysis
14.12.5 The SNS View
14.13 Renishaw plc
14.13.1 Company Overview
14.13.2 Financial
14.13.3 Products/ Services Offered
14.13.4 SWOT Analysis
14.13.5 The SNS View
14.14 Materialise NV
14.14.1 Company Overview
14.14.2 Financial
14.14.3 Products/ Services Offered
14.14.4 SWOT Analysis
14.14.5 The SNS View
14.15 Laser GmbH
14.15.1 Company Overview
14.15.2 Financial
14.15.3 Products/ Services Offered
14.15.4 SWOT Analysis
14.15.5 The SNS View
15. Competitive Landscape
15.1 Competitive Benchmarking
15.2 Market Share Analysis
15.3 Recent Developments
15.3.1 Industry News
15.3.2 Company News
15.3.3 Mergers & Acquisitions
16. Use Case and Best Practices
17. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
The High-Performance Liquid Chromatography Market size was valued at USD 4.8 billion in 2023 and is expected to grow to USD 7.83 billion by 2031 and grow at a CAGR of 6.30% over the forecast period of 2024-2031.
The Clinical Trial Management System Market Size was valued at USD 1.82 billion in 2023, and is expected to reach USD 5.49 billion by 2031 and grow at a CAGR of 14.8% over the forecast period 2024-2031.
Report Scope & Overview: The Infusion Pump Software Market Size was valued at USD 924.28 million in 2022 and is expected to reach USD 1660 million by 2030 and grow at a CAGR of 7.6% over the forecast period 2023-2030.
The 3D Ultrasound Market was estimated at USD 3.65 billion in 2022 and is poised to reach USD 6.17 billion in 2030 anticipated to expand at a compound annual growth rate of approx. CAGR of 6.8% for the forecast period of 2023-2030.
The Real World Evidence/RWE Solutions Market Size was projected at USD 43.22 billion in 2022, and is expected to increase at a CAGR of 7.8% from 2023 to 2030, reaching USD 78.83 billion by 2030.
The Antiepileptic Drugs Market size was estimated at USD 18.21 billion in 2023 and is expected to reach USD 26.30 billion by 2031 at a CAGR of 4.70% during the forecast period of 2024-2031.
Hi! Click one of our member below to chat on Phone