Alpha-1 Antitrypsin Deficiency Market Report Scope & Overview:
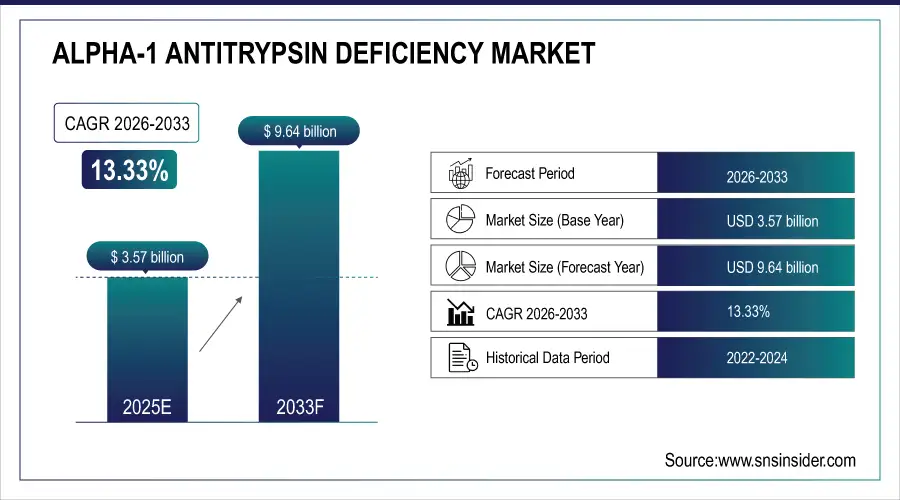
The Alpha-1 Antitrypsin Deficiency Market was valued at USD 3.57 billion in 2025E and is expected to reach USD 9.64 billion by 2032, growing at a CAGR of 13.33% from 2026-2033.
The Alpha-1 Antitrypsin Deficiency Market is growing due to increasing prevalence of genetic respiratory disorders and rising awareness among patients and healthcare providers. Advancements in augmentation therapies, inhalation treatments, and supportive care options are improving patient outcomes. Expansion of healthcare infrastructure, specialty clinics, and hospital-based treatment centers enhances accessibility. Additionally, government initiatives, insurance coverage, and investment in R&D for innovative therapies are driving adoption. Early diagnosis and improved treatment adherence further contribute to sustained market growth globally.
The U.S. Food and Drug Administration (FDA) has approved weekly intravenous treatment with human alpha-1 antitrypsin (A1-PI) to raise blood levels in severe AATD patients with emphysema.
AATD affects approximately 1 in 2,500 individuals globally, with higher prevalence in individuals of European descent. Many individuals with AATD, especially those with chronic obstructive pulmonary disease (COPD), remain undiagnosed, with studies suggesting up to 25% of COPD patients have never smoked yet are still affected by AATD.
Market Size and Forecast
-
Market Size in 2025: USD 3.57 Billion
-
Market Size by 2033: USD 9.64 Billion
-
CAGR: 13.33% from 2026 to 2033
-
Base Year: 2025E
-
Forecast Period: 2026–2033
-
Historical Data: 2022–2024
To Get more information On Alpha-1 Antitrypsin Deficiency Market - Request Free Sample Report
Alpha-1 Antitrypsin Deficiency Market Trends
-
Rising prevalence of genetic and respiratory disorders is driving the alpha-1 antitrypsin deficiency (AATD) market.
-
Growing adoption of augmentation therapy and enzyme replacement treatments is boosting market growth.
-
Increasing awareness and early diagnosis initiatives are improving patient identification and treatment rates.
-
Expansion of biopharmaceutical R&D and clinical trials is accelerating development of novel therapies.
-
Focus on personalized medicine and genetic testing is shaping therapeutic strategies.
-
Advancements in inhalation, gene therapy, and recombinant protein technologies are enhancing treatment options.
-
Collaborations between biotech firms, healthcare providers, and patient advocacy groups are driving innovation and market penetration.
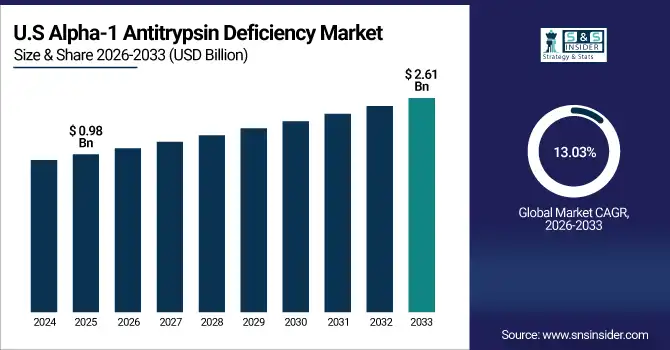
U.S. Alpha-1 Antitrypsin Deficiency Market was valued at USD 0.98 billion in 2025E and is expected to reach USD 2.61 billion by 2032, growing at a CAGR of 13.03% from 2026-2033.
The U.S. Alpha-1 Antitrypsin Deficiency Market is growing due to increasing disease awareness, early diagnosis, advanced therapies, and strong healthcare infrastructure, supported by government initiatives, insurance coverage, and ongoing R&D for innovative treatment options enhancing patient outcomes.
Alpha-1 Antitrypsin Deficiency Market Growth Drivers:
-
Technological advancements in therapies and supportive healthcare infrastructure are accelerating the Alpha-1 Antitrypsin Deficiency Market expansion globally
Innovations in intravenous augmentation therapies, inhalation treatments, and biologics are enhancing efficacy and safety profiles for AATD patients. Advanced healthcare infrastructure, including specialty clinics and home care services, allows broader patient access to these therapies. Integration of telemedicine and monitoring devices improves adherence, follow-ups, and patient management. Pharmaceutical investments in R&D and strategic collaborations enable faster clinical trials and regulatory approvals. These advancements make treatment more accessible, reliable, and effective, directly boosting adoption rates. Improved healthcare facilities combined with new therapeutic innovations are key enablers of sustained market growth and long-term competitiveness.
|
Company / Organization |
Initiative / Therapy (with Details) |
Updates |
|
Beam Therapeutics |
BEAM-302 (Base-Editing Gene Therapy): A base-editing therapy designed to correct the genetic mutation responsible for AATD. By repairing the defective gene, it aims to restore functional AAT protein levels and halt lung and liver damage. Represents one of the first applications of base editing in rare genetic liver disease. |
Positive initial Phase 1/2 data reported (March 2025). |
|
Wave Life Sciences |
RNA Editing Therapy: Uses endogenous RNA editing to repair faulty transcripts without altering DNA. Demonstrated the ability to restore functional AAT protein in patients, pioneering RNA editing as a therapeutic approach for genetic diseases. |
Two patients showed significant AAT protein production after a single dose (October 2024). |
|
Grifols |
Alpha-1 15% (Subcutaneous Formulation): A next-generation augmentation therapy offering subcutaneous delivery instead of intravenous infusion. Designed to improve patient convenience and adherence by allowing at-home administration. |
Completed second cohort enrollment in Phase 1/2 study (February 2025). |
|
Arrowhead Pharmaceuticals |
ARO-AAT (RNA Interference Therapy): An RNAi therapy that silences production of misfolded AAT in the liver, reducing toxic polymer accumulation and preventing fibrosis. Represents a disease-modifying approach for AATD liver disease. |
Received FDA Breakthrough Therapy Designation; Phase 2 SEQUOIA study fully enrolled. |
|
Gain Therapeutics |
Allosteric Small Molecule Regulators: Small molecules designed to restore normal folding and prevent toxic aggregation of AAT protein. Provides a potential oral therapy alternative supported by Eurostars and Innosuisse grants. |
Development supported since 2023 by Eurostars & Innosuisse funding. |
|
Vertex Pharmaceuticals |
Small Molecule Therapies: Research program aimed at correcting misfolding of the AAT protein in hepatocytes, addressing liver injury and cirrhosis risk in AATD. Still in preclinical stages, but represents a precision medicine approach. |
Actively conducting research. |
|
Alpha-1 Foundation |
AlphaDetect (Screening Initiative): A nonprofit program providing free genetic testing and centralized screening to improve early diagnosis and integrate AATD testing into routine lung and liver care. |
Launched in August 2025. |
Alpha-1 Antitrypsin Deficiency Market Restraints:
-
Low disease awareness and delayed diagnosis hinder rapid adoption and expansion of Alpha-1 Antitrypsin Deficiency Market globally
Many patients remain undiagnosed due to the rarity of the disorder and lack of routine genetic screening. Symptoms often mimic other respiratory conditions, leading to delayed identification and treatment. Limited patient and physician awareness reduces early intervention, slowing therapy adoption. Geographic disparities in healthcare infrastructure exacerbate these challenges, particularly in underdeveloped regions. These factors prevent optimal utilization of available therapies, reducing market potential. Awareness campaigns, early screening programs, and educational initiatives are critical to overcoming these obstacles, but until widespread knowledge and diagnostic improvements occur, market growth remains constrained by delayed treatment initiation and low patient engagement.
Alpha-1 Antitrypsin Deficiency Market Opportunities:
-
Growing focus on gene therapies and personalized medicine creates significant opportunities in Alpha-1 Antitrypsin Deficiency Market
Advancements in gene editing, RNA-based therapies, and personalized treatment approaches offer potential curative solutions for AATD, beyond symptomatic management. These innovative therapies can improve patient outcomes, reduce long-term healthcare costs, and address the underlying genetic cause. Pharmaceutical companies and biotech startups are investing heavily in R&D for next-generation treatments. Clinical trials and regulatory support are expanding, allowing accelerated adoption upon approval. The shift toward precision medicine creates opportunities for differentiated products, strategic partnerships, and novel therapeutic pipelines. These developments position the market for long-term growth and higher patient penetration globally.
Alpha-1 Antitrypsin Deficiency Market Segment Highlights
-
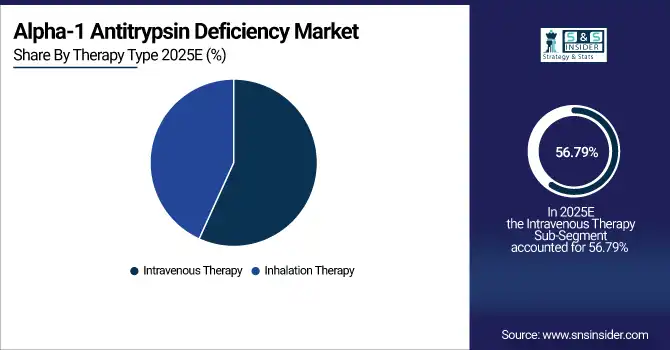
By Therapy Type, Intravenous Therapy dominated with ~56.79% share in 2025; Inhalation Therapy fastest growing (CAGR).
-
By Patient Age Group, Adult dominated with ~74% share in 2025; Pediatric fastest growing (CAGR).
-
By Product Type, Augmentation Therapy dominated with ~44% share in 2025; Oxygen Therapy fastest growing (CAGR).
-
By Application, Hospitals dominated with ~52% share in 2025; Specialty Clinics fastest growing (CAGR).
Alpha-1 Antitrypsin Deficiency Market Segment Analysis
By Therapy Type, Intravenous Therapy dominated in 2025; Inhalation Therapy expected fastest growth 2026–2033 due to non-invasive, patient-friendly delivery.
Intravenous Therapy segment dominated the market in 2025 due to its proven efficacy in delivering augmentation therapy directly to patients. Hospitals and specialized centers prefer this route for reliable and controlled dosing. The ability to rapidly improve alpha-1 antitrypsin levels in blood and manage respiratory symptoms makes it the preferred treatment method.
Inhalation Therapy segment is expected to grow fastest from 2026 to 2033 as it offers non-invasive administration, improved patient convenience, and better adherence. Its suitability for home care and chronic management of respiratory symptoms drives adoption. Increasing focus on patient-friendly treatments and ongoing clinical advancements are accelerating market growth for inhalation-based therapies.
By Patient Age Group, Adult segment led in 2025; Pediatric segment projected fastest growth 2026–2033 with early diagnosis and specialized care adoption.
Adult segment dominated the market in 2025 because the majority of diagnosed AATD patients are adults, with higher prevalence of COPD and liver complications. Adults are more likely to receive regular therapies, including augmentation and supportive treatments, leading to higher market revenue. Established treatment protocols and healthcare infrastructure for adult care further strengthen dominance.
Pediatric segment is expected to grow fastest from 2026 to 2033 due to increasing awareness of early diagnosis and treatment benefits. Advancements in pediatric formulations and specialized care programs are expanding adoption. Early intervention helps prevent disease progression, making pediatric-focused therapies a rapidly growing segment in the global market.
By Product Type, Augmentation Therapy dominated in 2025; Oxygen Therapy expected fastest growth 2026–2033 driven by home-based COPD and hypoxemia management.
Augmentation Therapy segment dominated the market in 2025 as it directly addresses alpha-1 antitrypsin deficiency, improving lung function and slowing disease progression. Its established clinical efficacy, long-standing use, and insurance coverage make it the preferred treatment option, contributing to higher revenue compared to other therapies.
Oxygen Therapy segment is expected to grow fastest from 2026 to 2033 as increasing COPD prevalence and hypoxemia management drive demand. Home-based oxygen solutions and portable devices improve patient quality of life. Rising awareness and adoption of supportive therapies for symptomatic management contribute to the segment’s rapid growth.
By Application, Hospitals led in 2025; Specialty Clinics projected fastest growth 2026–2033 due to personalized, outpatient-focused patient care models.
Hospitals segment dominated the market in 2025 due to the availability of advanced infrastructure, trained personnel, and specialized care units capable of administering complex treatments like intravenous augmentation therapy. Centralized patient management, monitoring, and immediate access to supportive care make hospitals the primary treatment setting for AATD patients.
Specialty Clinics segment is expected to grow fastest from 2026 to 2033 as these centers provide focused care, personalized treatment plans, and innovative therapies. The rise of outpatient management programs and patient-centric models encourages adoption, offering convenience and improved adherence, driving faster growth in specialty clinics compared to traditional hospital settings.
Alpha-1 Antitrypsin Deficiency Market Regional Analysis
North America Alpha-1 Antitrypsin Deficiency Market Insights
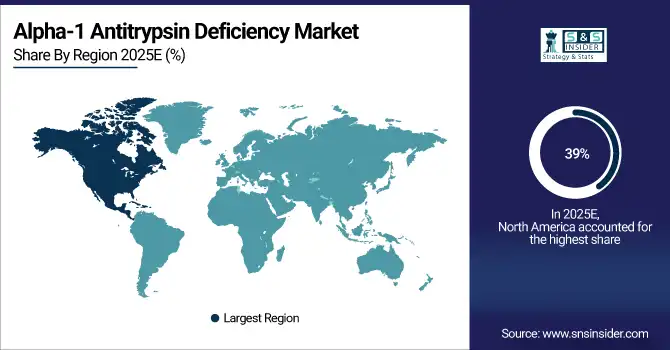
North America dominated the Alpha-1 Antitrypsin Deficiency Market in 2025 with the highest revenue share of about 39% due to advanced healthcare infrastructure, widespread availability of specialized therapies, and high disease awareness. Strong government support, well-established patient registries, and reimbursement policies facilitate access to augmentation and supportive treatments. Additionally, extensive R&D activities and presence of major pharmaceutical companies further strengthen market dominance in the region.
Get Customized Report as per Your Business Requirement - Enquiry Now
Asia Pacific Alpha-1 Antitrypsin Deficiency Market Insights
Asia Pacific is expected to grow at the fastest CAGR of about 15.855% from 2026 to 2033 due to increasing awareness, improving healthcare infrastructure, and rising prevalence of respiratory disorders. Expansion of specialty clinics, growing patient diagnosis, and government initiatives to support rare disease management are driving adoption. Increasing investments by global pharmaceutical players and improved access to therapies in emerging countries are accelerating market growth in the region.
Europe Alpha-1 Antitrypsin Deficiency Market Insights
Europe in the Alpha-1 Antitrypsin Deficiency Market is experiencing steady growth due to well-established healthcare infrastructure, widespread availability of augmentation and supportive therapies, and high disease awareness. Government initiatives and reimbursement policies facilitate patient access to treatments. Increasing diagnosis rates, presence of major pharmaceutical players, and continuous investment in R&D for advanced therapies further drive market expansion. Collaborative efforts between patient advocacy groups and healthcare providers enhance early detection and treatment adoption across the region.
Middle East & Africa and Latin America Alpha-1 Antitrypsin Deficiency Market Insights
The Middle East & Africa and Latin America in the Alpha-1 Antitrypsin Deficiency Market are emerging regions with growing potential due to improving healthcare infrastructure, increasing disease awareness, and rising adoption of advanced therapies. Government initiatives and investment in rare disease management are supporting market growth. Expansion of specialty clinics, better diagnostic facilities, and entry of global pharmaceutical companies are enhancing accessibility and treatment availability, driving gradual market penetration across these regions.
Alpha-1 Antitrypsin Deficiency Market Competitive Landscape:
Grifols S.A.
Grifols S.A. is a global healthcare company specializing in plasma-derived therapies, hospital products, and diagnostic solutions. The company focuses on innovative treatments for rare diseases, including Alpha-1 Antitrypsin Deficiency (AATD), aiming to improve patient outcomes through safer, more convenient therapies. Grifols leverages advanced clinical research and subcutaneous administration technologies to expand at-home treatment options, enhancing accessibility and quality of life for patients requiring lifelong therapy.
-
November 15, 2023: Grifols concluded Cohort 1 of the Alpha-1 15% clinical trial, moving forward into Cohort 2 to evaluate the first subcutaneous dosing option for AATD patients.
-
February 18, 2025: Grifols completed enrollment of the second cohort in its first-in-human clinical study of Alpha-1 15%, aiming to provide patients with a more convenient home-administered therapy.
Takeda Pharmaceutical Company Limited
Takeda Pharmaceutical Company Limited is a global biopharmaceutical leader dedicated to delivering innovative treatments across rare diseases, oncology, and vaccines. Takeda emphasizes patient-centric solutions, streamlining therapeutic administration, and collaborating on clinical research initiatives to accelerate innovation. Its Alpha-1 Proteinase Inhibitor therapies aim to improve the infusion experience, optimize dosing, and enhance patient convenience, particularly for those with chronic conditions like AATD.
-
May 6, 2025: Takeda contributed Phase 2 clinical trial data to the Critical Path for Alpha-1 (CPA-1) consortium, accelerating innovation in AATD therapies.
-
March 11, 2025: Takeda announced approval of larger vial sizes for GLASSIA® (Alpha-1 Proteinase Inhibitor), improving the infusion experience for patients requiring multiple vials per treatment.
Kamada Pharmaceuticals
Kamada Pharmaceuticals is an Israeli biopharmaceutical company focusing on specialty and orphan drugs. The company develops inhaled and intravenous therapies for rare diseases, including AATD, with a strong commitment to long-term clinical studies and patient access. Kamada’s therapeutic pipeline emphasizes continuous evaluation, safety, and real-world applicability to enhance patient outcomes while advancing treatment options in pulmonary and systemic rare disorders.
April 1, 2013: Kamada published an interim report for its pivotal clinical trial on inhaled AAT therapy for AATD, showing patient adoption beyond the trial timeframe.
GlaxoSmithKline (GSK)
GlaxoSmithKline (GSK) is a leading global biopharmaceutical company with a focus on innovative medicines, vaccines, and specialty therapies. In the AATD space, GSK leverages RNA-editing technologies to address underlying genetic defects, aiming for disease-modifying treatments. Their R&D emphasizes precision medicine and RNA therapeutics, enabling the development of targeted therapies for rare genetic disorders and enhancing long-term patient health outcomes.
-
July 2024: GSK’s pipeline includes GSK5462688, an RNA-editing oligonucleotide targeting AATD, designed to correct the genetic defect underlying the condition.
Sanofi S.A.
Sanofi S.A. is a global biopharmaceutical company focused on rare diseases, vaccines, and specialty care. The company strengthens its pipeline through strategic acquisitions, enhancing access to recombinant protein therapies and expanding capabilities in rare genetic disorders. Sanofi’s focus on AATD includes innovative recombinant AAT therapies to provide patients with new treatment modalities that improve disease management and quality of life.
-
July 2024: Sanofi acquired Inhibrx for $1.7 billion, gaining access to INBRX-101, a recombinant AAT therapy for AATD, bolstering its rare disease portfolio.
Key Players
Some of the Alpha-1 Antitrypsin Deficiency Market Companies
-
Grifols S.A.
-
Takeda Pharmaceutical Company Limited
-
CSL Behring
-
Kamada Pharmaceuticals
-
GlaxoSmithKline plc
-
AstraZeneca Plc
-
Boehringer Ingelheim International GmbH
-
Pfizer Inc.
-
Arrowhead Pharmaceuticals, Inc.
-
Vertex Pharmaceuticals Incorporated
-
Mereo Biopharma Group plc
-
Dicerna Pharmaceuticals, Inc.
-
Inhibrx, Inc.
-
Teva Pharmaceutical Industries Ltd.
-
LFB Biomedicaments S.A.
-
Alnylam Pharmaceuticals, Inc.
-
Sanofi S.A.
-
CHIESI Farmaceutici S.p.A.
-
Epicrispr Biotechnologies, Inc.
-
Intellia Therapeutics, Inc.
| Report Attributes | Details |
|---|---|
| Market Size in 2025E | USD 3.57 Billion |
| Market Size by 2033 | USD 9.64 Billion |
| CAGR | CAGR of 13.33% From 2026 to 2033 |
| Base Year | 2025 |
| Forecast Period | 2026-2033 |
| Historical Data | 2022-2024 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product Type (Augmentation Therapy, Bronchodilators, Corticosteroids, Oxygen Therapy) • By Application (Hospitals, Specialty Clinics, Pharmacies) • By Therapy Type (Intravenous Therapy, Inhalation Therapy) • By Patient Age Group (Adult, Pediatric) |
| Regional Analysis/Coverage | North America (US, Canada), Europe (Germany, UK, France, Italy, Spain, Russia, Poland, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Australia, ASEAN Countries, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Mexico, Colombia, Rest of Latin America). |
| Company Profiles | Grifols S.A., Takeda Pharmaceutical Company Limited, CSL Behring, Kamada Pharmaceuticals, GlaxoSmithKline plc, AstraZeneca Plc, Boehringer Ingelheim International GmbH, Pfizer Inc., Arrowhead Pharmaceuticals, Inc., Vertex Pharmaceuticals Incorporated, Mereo Biopharma Group plc, Dicerna Pharmaceuticals, Inc., Inhibrx, Inc., Teva Pharmaceutical Industries Ltd., LFB Biomedicaments S.A., Alnylam Pharmaceuticals, Inc., Sanofi S.A., CHIESI Farmaceutici S.p.A., Epicrispr Biotechnologies, Inc., Intellia Therapeutics, Inc. |

