Biotech CRO Market Report Scope & Overview:
Get More Information on Biotech CRO (Contract Research Organization) Market - Request Sample Report
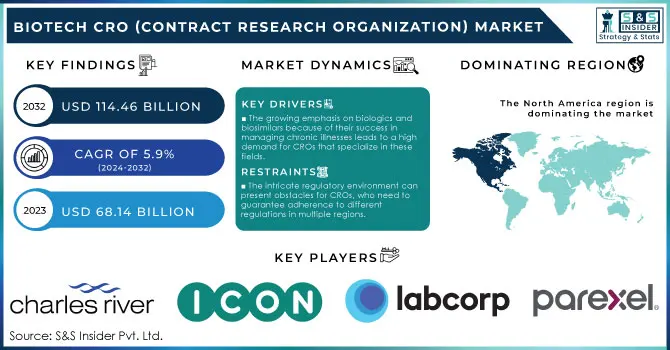
The Biotech CRO (Contract Research Organization) Market Size was valued at USD 68.14 billion in 2023 and is expected to reach USD 114.46 billion by 2032, growing at a CAGR of 5.9% from 2024-2032.
The complexity of drug development is increasing due to advancements in areas such as biologics, cell and gene therapies, and personalized medicine, leading to this growth. The increasing need for biologics has driven growth in the global biologics market, projected to reach over 114 billion by 2032, leading to a higher demand for specialized CRO services.
Outsourcing R&D to CROs is now a common practice, with around 50-60% of biotech firms choosing to outsource their research and clinical trial tasks. This is especially noticeable in small and mid-sized biotech companies, a lot of which do not have the necessary resources to manage complicated trials internally. Moreover, there is a growing trend in global clinical trials, with almost 40% of trials currently taking place in various countries, leading to an increased need for CROs to oversee the regulatory and operational components of these trials.
Additionally, the industry is being transformed by the incorporation of digital technologies such as artificial intelligence (AI) and machine learning (ML). A new report from Nature Digital Medicine found that using AI for patient recruitment can reduce clinical trial expenses by 70% and speed up timelines by 40%. The typical expense for phase 1, 2, and 3 clinical trials in various therapeutic fields is approximately USD 4 million, USD 13 million, and USD 20 million, respectively. Phase 3 pivotal trials for recently approved medications by the FDA in the US have a median cost of USD 41,117 per patient. By the end of the decade, it is anticipated that 25-30% of all trials will be decentralized, utilizing telemedicine and remote monitoring. This will drive the need for CROs with these specific capabilities.
Biotech CRO Market Dynamics
Drivers
-
The growing emphasis on biologics and biosimilars because of their success in managing chronic illnesses leads to a high demand for CROs that specialize in these fields.
Biologics are intricate drugs sourced from living beings, such as proteins, monoclonal antibodies, and gene therapies. The rising occurrence of chronic illnesses like cancer, autoimmune disorders, and rare genetic conditions is increasing the need for this advanced treatment.
In 2023, the FDA granted approval to 55 new treatments, marking the second-highest number within the past three decades. This consisted of 17 biologics, with 12 of them being monoclonal antibodies (mAbs).
Biotech firms need specific skills in clinical trials, regulatory submissions, and manufacturing processes as they increase their investment in biologics development. CROs with expertise in biologics can offer important services like preclinical testing, overseeing clinical trials, and ensuring regulatory compliance, allowing them to gain a bigger market portion. The increasing approval rates of biologics are reinforcing this trend, resulting in more pipeline projects and a greater demand for CRO partnerships.
-
Biotech companies are turning to CROs more and more for their R&D needs to lower expenses, enhance productivity, and tap into expert knowledge.
Numerous biotech companies are more frequently subcontracting their research and development (R&D) operations to CROs as a tactic to lower expenses, manage risks, and improve effectiveness. This pattern has sped up, especially for small to medium-sized biotech firms without the required infrastructure or resources for internal R&D. Outsourcing enables biotech companies to utilize specialized expertise, cutting-edge technologies, and established operational structures provided by CROs. This helps biotech companies not only streamline their development processes but also concentrate on their main areas of expertise like drug discovery and strategic planning. Consequently, there is an increasing need for CRO services, which has resulted in CROs expanding their services and reach geographically, establishing them as essential partners in the biotech development industry.
Restraint
-
The intricate regulatory environment can present obstacles for CROs, who need to guarantee adherence to different regulations in multiple regions.
The biotech market faces extensive regulation from entities like the FDA, EMA, and other worldwide regulatory organizations. These guidelines guarantee the safety, effectiveness, and quality of medications and treatments, especially for biological and gene therapies. Adhering to these regulations is essential but may be intricate and demanding of time.
CROs need to maneuver through the complex regulatory environment when carrying out clinical trials and submitting data for drug approval. Staying informed about changing regulations in various regions is difficult for CROs. Failure to comply can result in trial results or drug application rejections, as well as costly revisions or delays. Moreover, dealing with varying regulations across countries can add complexity to international research studies. An in-depth understanding of regulations raises operational expenses, which could be transferred to biotech clients, possibly reducing the attractiveness of CRO services for small biotech companies with financial restrictions.
Biotech CRO Market Segmentation Overview
By Type
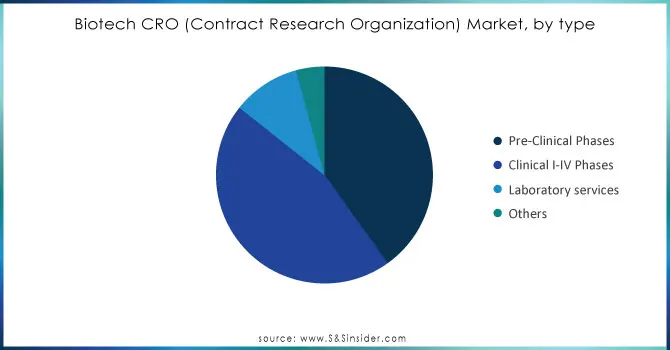
The Biotech CRO market is divided into Pre-Clinical Phases, clinical services, laboratory services, and others. The market was mainly led by the Clinical Services segment in 2023, with around 45% of the total market share. The reason for this dominance is the growing attention of market participants to improve these services for chronic conditions, which encompassed phase 1, phase 2, phase 3, and phase 4 trials. In December 2022, Phastar joined Lean Life Science’s Oncology Development Programme (ODP2) to assist in discovering and backing R&D for academics and early-stage companies. Around 40% of the total market share in 2023 was accounted for by the pre-clinical phases segment, This increase is fueled by the rise of small and medium-sized pharmaceutical and biotech firms dedicated to creating innovative treatments. Furthermore, there is an expectation for laboratory services to experience significant growth with a CAGR of around 9% in the forecasted period. This growth is driven by rising demand from healthcare companies lacking resources who are looking to delegate specialized tasks.
Need Any Customization Research On Biotech CRO (Contract Research Organization) Market - Inquiry Now
By Application
The Biotech CRO market is divided by application into different areas such as oncology, neurology, cardiology, infectious diseases, metabolic disorders, respiratory disorders, and more. In the year 2023, the oncology sector became the leading influence in the industry by 35%, as life science firms increasingly concentrated on creating new therapies for cancer care. Furthermore, the market in 2023 was predominantly divided the share by various categories. This increase is due to the increasing prevalence of different illnesses, including digestive diseases, nutritional deficiencies, and respiratory disorders. Moreover, the substantial increase in financial support from pharmaceutical and biotechnology companies has greatly aided in the advancement of treatments for these conditions, leading to significant growth in this sector.
Biotech CRO Market Regional Analysis
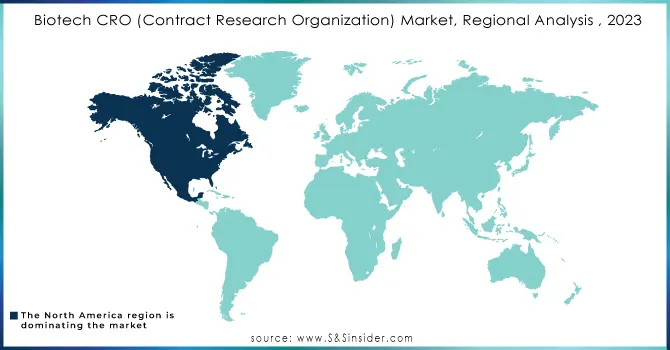
In 2023, North America remains the top player in the Biotech CRO market, maintaining its leading position with the largest market share. The reason for this dominance is mainly because of the strong biotechnology framework in the United States, where many top biotech and pharmaceutical companies invest significantly in research and development. Furthermore, significant investment in research and development, specifically in cancer treatment and other medical areas, has established North America as a top player in the industry, holding around 60% of the worldwide Biotech CRO market share. An advantageous regulatory landscape, supported by effective procedures implemented by agencies such as the FDA, also boosts the region's appeal for undertaking clinical trials and drug development.
On the other hand, Asia-Pacific is identified as the market experiencing the most rapid expansion in Biotech CRO services, expecting an 8% CAGR from 2023 to 2032. The rise in this development can be credited to the rise of a dynamic biotechnology environment in nations like China, India, and Japan, bolstered by government efforts and growing funding in healthcare studies. The area provides cost benefits, such as affordable workforce and materials, that encourage pharmaceutical and biotech firms to contract out their R&D work to nearby CROs. Moreover, the increasing number of clinical trials in the Asia-Pacific region, especially in fields such as oncology, infectious diseases, and metabolic disorders, demonstrates a stronger dedication to creating new treatments for the region's healthcare challenges.
Key Market Players in Biotech CRO Market
-
Charles River Laboratories (Preclinical safety testing, Biologics testing solutions)
-
ICON plc (Clinical trial management, Decentralized clinical trial)
-
Labcorp Drug Development (Biomarker discovery and development, Central laboratory services)
-
Parexel (Clinical data management, Regulatory consulting)
-
IQVIA (Real-world evidence solutions, AI-based clinical trial design)
-
Syneos Health (Clinical development services, Medical affairs solutions)
-
Medpace (Full-service clinical trial management, Regulatory submission support)
-
Covance (Toxicology studies, Drug metabolism and pharmacokinetics (DMPK) services)
-
PPD (Thermo Fisher Scientific) (Phase I-IV clinical trial services, Patient recruitment services
-
PRA Health Sciences (Clinical trial outsourcing, Post-marketing surveillance)
-
WuXi AppTec (Biologics CMC services, Gene therapy process development)
-
Pharmaron (Integrated drug discovery services, Preclinical safety assessment)
-
Eurofins Scientific (Genomic testing for personalized medicine, Pharmacokinetics and ADME
-
Frontage Laboratories (Bioanalytical services, Clinical pharmacology)
-
KCR (Patient recruitment and retention, Data management and analysis)
-
Worldwide Clinical Trials (Neuroscience clinical research, Cardiovascular clinical trials)
-
Clinipace (Clinical trial project management, Regulatory and strategic development)
-
SGS Life Sciences (Biomarker and clinical bioanalysis services, Biopharmaceutical testing)
-
Novotech (Oncology clinical trials, Early-phase clinical development)
-
Tigermed (Biostatistics and data management, Regulatory affairs and compliance)
Key Market Suppliers:
-
Thermo Fisher Scientific
-
PerkinElmer
-
Abbott Laboratories
-
Agilent Technologies
-
MilliporeSigma
-
Sartorius AG
-
Roche Diagnostics
-
Lonza Group
-
GE Healthcare Life Sciences
-
Bio-Rad Laboratories
Recent Developments
-
In June 2023, ICON plc revealed the introduction of its extended range of decentralized clinical trial (DCT) solutions, utilizing digital technologies to enhance patient participation and involvement in clinical trials. This project seeks to simplify the trial process by integrating remote patient monitoring, telehealth services, and mobile health technologies. The new solutions aim to meet the increasing need for trial designs that are more adaptable and focused on patients, improving the rates of recruitment and retention.
-
In April 2023, Charles River Laboratories finalized the purchase of Labcorp Drug Development, bolstering its presence in preclinical and clinical research. This purchase is anticipated to improve Charles River's expertise in drug development and biologics testing solutions, allowing it to provide a broader range of services to customers. Labcorp's vast resources and expertise combined with Charles River will enhance support for biotech firms in their research and development endeavors.
-
In August 2023, Parexel launched innovative AI-based methods to enhance the planning of clinical trials and the recruitment of patients. These advancements utilize machine learning algorithms to examine large quantities of data, assisting sponsors in identifying appropriate locations and patient groups with greater efficiency. This decision is in line with Parexel's plan to improve the effectiveness of clinical trials and shorten the time-to-market for new treatments, addressing the growing need for quicker and more efficient drug development.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 68.14 Billion |
| Market Size by 2032 | US$ 114.46 Billion |
| CAGR | CAGR of 5.9% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Type (Pre-Clinical Phases,Clinical I-IV Phases, Laboratory services, Others) • By Application (Oncology, Neurology, Cardiology, Infectious Disease, Respiratory Disorder, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe [Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Charles River Laboratories, ICON plc, Labcorp Drug Development, Parexel, IQVIA, Syneos Health, Medpace, Covance, PPD (Thermo Fisher Scientific), PRA Health Sciences, WuXi AppTec, Pharmaron, Eurofins Scientific, Frontage Laboratories, KCR, Worldwide Clinical Trials, Clinipace, SGS Life Sciences, Novotech, Tigermed. |
| Key Drivers | • The growing emphasis on biologics and biosimilars because of their success in managing chronic illnesses leads to a high demand for CROs that specialize in these fields. • Biotech companies are turning to CROs more and more for their R&D needs to lower expenses, enhance productivity, and tap into expert knowledge. |
| Restraints | • The intricate regulatory environment can present obstacles for CROs, who need to guarantee adherence to different regulations in multiple regions. |

