Neonatal Respiratory Care Devices Market Report Scope & Overview:
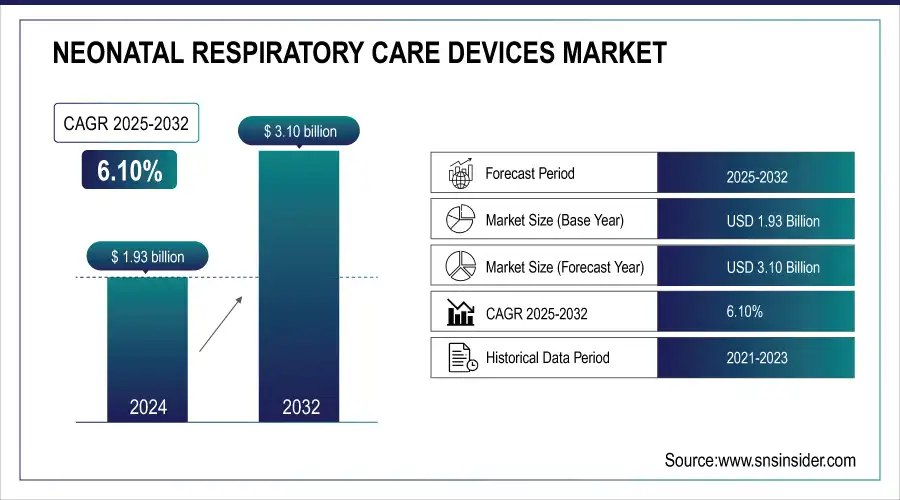
The Neonatal Respiratory Care Devices Market size was valued at USD 1.93 billion In 2025E & is estimated to reach USD 3.10 billion by 2033 and increase at a compound annual growth rate of 6.10% between 2026 and 2033.
The Neonatal Respiratory Care Devices Market refers to the market for medical devices that are specifically designed to provide respiratory support and care to newborn infants, particularly those who are born prematurely or with respiratory issues. These devices are crucial for managing various respiratory conditions and ensuring proper oxygenation and ventilation in neonates, as their underdeveloped lungs and respiratory systems can pose significant challenges.
Market Size and Forecast:
-
Neonatal Respiratory Care Devices Market Size in 2025E: USD 1.93 Billion
-
Neonatal Respiratory Care Devices Market Size by 2033: USD 3.10 Billion
-
CAGR: 6.10% from 2026 to 2033
-
Base Year: 2025
-
Forecast Period: 2026–2033
-
Historical Data: 2022–2024
Get More Information on Neonatal Respiratory Care Devices Market - Request Sample Report
Key Neonatal Respiratory Care Devices Market Trends
-
Increasing adoption of non-invasive ventilation (NIV) techniques, such as CPAP and HFNC, to minimize lung injury and improve neonatal outcomes.
-
Growing integration of AI and machine learning algorithms for real-time respiratory monitoring and early distress detection in NICUs.
-
Rising demand for portable and compact respiratory devices to support neonatal care in low-resource and remote settings.
-
Shift toward disposable and single-use respiratory interfaces to reduce infection risks and cross-contamination in NICUs.
-
Expanding use of tele-neonatology and remote patient monitoring for post-discharge respiratory management.
-
Development of wearable biosensors and miniaturized monitoring systems for continuous, non-invasive respiratory assessment.
Neonatal Respiratory Care Devices Market Growth Drivers
- Growing Prevalence of Preterm Births and Respiratory Disorders Boosts Neonatal Respiratory Care Devices Market Growth
The rising incidence of premature births and associated respiratory complications such as neonatal respiratory distress syndrome (NRDS) and bronchopulmonary dysplasia is driving the demand for advanced neonatal respiratory care devices. According to the WHO, around 15 million babies are born preterm each year, creating a significant need for ventilators, CPAP systems, and oxygen therapy devices. Technological advancements, such as AI-driven monitoring and non-invasive ventilation systems, enhance treatment precision while minimizing lung damage. For instance, in June 2024, Philips introduced an updated non-invasive CPAP platform designed for fragile neonates, improving oxygen delivery and comfort levels across NICUs worldwide.
For example, hospitals in the United States and India have reported a 30% increase in the adoption of non-invasive neonatal ventilation systems due to higher preterm birth rates. This surge in demand has encouraged manufacturers like Philips and Fisher & Paykel to expand product lines targeting neonatal respiratory stabilization.
Neonatal Respiratory Care Devices Market Restraints
- High Equipment Costs and Maintenance Requirements Restrain Neonatal Respiratory Care Devices Market Growth
The significant cost of neonatal ventilators, CPAP systems, and oxygen therapy devices acts as a key barrier to market adoption, particularly in low- and middle-income countries. High procurement costs, coupled with maintenance, calibration, and training expenses, limit the accessibility of advanced respiratory care technologies. Moreover, the shortage of skilled neonatal care professionals further challenges effective equipment utilization. Public hospitals often struggle with limited budgets, slowing the pace of technology integration. The lack of cost-effective financing options and reimbursement policies also reduces the adoption of high-performance neonatal respiratory systems across emerging markets.
For instance, healthcare centers in sub-Saharan Africa and parts of Southeast Asia rely heavily on donor programs or refurbished devices, restricting consistent quality care. Limited funding has delayed the installation of advanced CPAP and ventilation units in several government-run neonatal intensive care units.
Neonatal Respiratory Care Devices Market Opportunities
- Integration of AI and Smart Monitoring Systems Creates New Opportunities in Neonatal Respiratory Care Devices Market
The incorporation of artificial intelligence (AI) and IoT-enabled systems in neonatal respiratory care is opening new avenues for growth. AI algorithms enhance real-time monitoring, predict respiratory failure, and automate ventilator settings to minimize human error. The integration of predictive analytics helps clinicians make faster decisions, improving neonatal survival rates. In March 2025, Masimo Corporation launched an AI-powered neonatal monitoring platform that provides early distress alerts based on oxygen saturation and respiratory pattern analysis. These innovations are expected to accelerate the adoption of smart respiratory devices in hospitals worldwide, improving patient outcomes and clinical efficiency.
For example, NICUs in Germany and Japan adopting AI-integrated monitoring systems have reported a 20% improvement in early detection of hypoxia and apnea episodes. Such smart technologies are setting a new benchmark for precision and proactive neonatal respiratory care.
Neonatal Respiratory Care Devices Market Segment Analysis
By Product Type
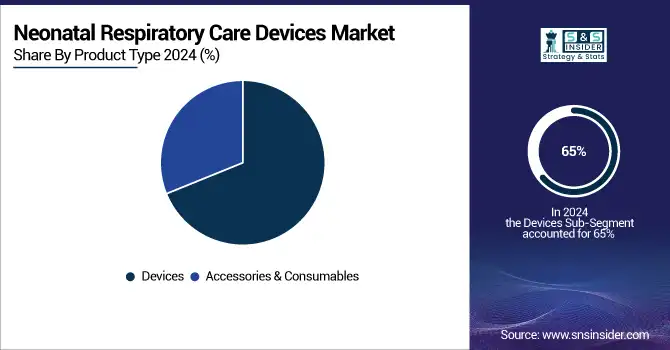
In 2025, Devices dominate the Neonatal Respiratory Care Devices Market with a 65% share, driven by their critical role in providing continuous respiratory support, reducing neonatal mortality, and improving outcomes in NICUs globally. Accessories & Consumables hold 35%, supporting efficient operation of devices through masks, tubing, filters, and other essential components. Increasing adoption of non-invasive ventilation systems and advanced ventilators in hospitals and specialty clinics fuels demand for both devices and supporting consumables, ensuring consistent and safe neonatal respiratory care.
By End-User
Hospitals account for the largest share at 70% in 2025, owing to their extensive NICU setups, advanced infrastructure, and ability to provide comprehensive neonatal care. Specialty Clinics hold 30%, catering to targeted neonatal care services and offering specialized respiratory support. The preference for hospitals is driven by higher patient volumes, integration of advanced devices, and the need for continuous monitoring, whereas clinics focus on cost-effective, portable, and less resource-intensive respiratory solutions, supporting the market’s overall growth and adoption trends.
Neonatal Respiratory Care Devices Market Regional Analysis
North America Neonatal Respiratory Care Devices Market Insights
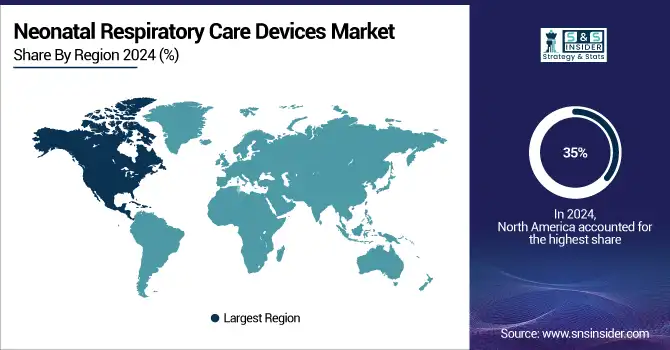
North America leads the global neonatal respiratory care devices market with a 35% share in 2025, driven primarily by the U.S. and Canada. Growth is supported by advanced healthcare infrastructure, high NICU bed capacity, and widespread adoption of cutting-edge respiratory technologies. Government initiatives promoting neonatal health, combined with R&D investments and collaborations between device manufacturers and hospitals, enhance market penetration. Increasing incidence of preterm births and focus on improving neonatal survival rates further drive adoption of ventilators, CPAP systems, and oxygen therapy devices across hospitals and specialty clinics.
Get Customized Report as per Your Business Requirement - Request For Customized Report
Europe Neonatal Respiratory Care Devices Market Insights
Europe accounts for 27% of the market, with Germany, the UK, and France leading adoption. The region benefits from robust regulatory frameworks, high-quality healthcare infrastructure, and strong emphasis on neonatal care standards. Investments in NICU modernization, neonatal training programs, and integration of advanced respiratory monitoring systems accelerate device adoption. Collaboration between hospitals, research institutions, and technology providers facilitates the deployment of innovative solutions, while initiatives to improve preterm care and reduce neonatal mortality rates continue to boost market growth across Western Europe.
Asia-Pacific Neonatal Respiratory Care Devices Market Insights
Asia-Pacific holds a 23% share in 2025, driven by China, Japan, South Korea, and India. Rapid expansion of healthcare infrastructure, rising preterm birth rates, and growing awareness of neonatal respiratory care are fueling market growth. Government support for hospital modernization, adoption of advanced NICU equipment, and rising healthcare spending contribute to increased deployment of ventilators, CPAP devices, and oxygen therapy systems. The region is witnessing fast adoption of AI-enabled monitoring and non-invasive ventilation technologies, positioning Asia-Pacific as one of the fastest-growing markets globally.
Latin America Neonatal Respiratory Care Devices Market Insights
Latin America represents 8% of the global market, with Brazil and Mexico leading adoption. Growth is supported by modernization of hospital facilities, expansion of NICU capacities, and increasing awareness of neonatal respiratory disorders. However, limited healthcare budgets and low technological readiness in certain countries restrict broader adoption. Partnerships with international device manufacturers and donor-funded programs help improve access to advanced respiratory care technologies, gradually enhancing market penetration.
Middle East & Africa (MEA) Neonatal Respiratory Care Devices Market Insights
MEA holds 7% of the global market, with the UAE, Saudi Arabia, and South Africa driving growth through investments in modern healthcare infrastructure and neonatal intensive care units. While regulatory challenges and scarcity of trained neonatal specialists pose hurdles, rising preterm birth rates, urban healthcare development, and adoption of innovative respiratory devices are expected to boost market growth across hospitals and specialized neonatal centers in the region.
Neonatal Respiratory Care Devices Market Competitive Landscape
Ambu A/S
Ambu A/S is a Denmark-based global company specializing in single-use and reusable medical devices, including advanced airway management and neonatal respiratory care solutions.
-
In May 2024, Ambu expanded its SureSight™ Connect System by introducing five new pediatric blades, enhancing airway management capabilities and improving neonatal care outcomes in hospitals and specialty clinics.
Cardinal Health, Inc.
Cardinal Health, Inc. is a U.S.-based healthcare services and products company offering medical devices, surgical solutions, and neonatal care technologies.
-
In August 2023, Cardinal Health launched the NTrainer™ System 2.0, an FDA Class II biofeedback device that helps premature newborns develop non-nutritive sucking skills, supporting early oral feeding and improved neonatal outcomes.
Koninklijke Philips N.V.
Koninklijke Philips N.V. is a Netherlands-based global technology company providing healthcare solutions, including neonatal monitoring, respiratory therapy, and sleep care devices.
-
In October 2024, Philips announced its ongoing expansion of neonatal respiratory care solutions outside the U.S., introducing next-generation ventilation devices, oxygen therapy systems, and monitoring solutions for hospitals and NICUs.
Neonatal Respiratory Care Devices Market Key Players
- Ambu A/S
- Cardinal Health
- Koninklijke Philips N.V.
- Drägerwerk AG & Co. KGaA
- Medtronic plc
- GE Healthcare Technologies Inc.
- Becton, Dickinson and Company (BD)
- Vyaire
- Natus Medical Incorporated
- Utah Medical Products, Inc.
- Fisher & Paykel Healthcare Corporation Limited
- ResMed Inc.
- Masimo Corporation
- Getinge AB
- Hamilton Medical AG
- Air Liquide Medical Systems
- Sechrist Industries, Inc.
- Inspiration Healthcare Group plc
- ICU Medical, Inc.
- Nihon Kohden Corporation
| Report Attributes | Details |
| Market Size in 2025E | US$ 1.93 Bn |
| Market Size by 2033 | US$ 3.10 Bn |
| CAGR | CAGR of 6.10 % From 2026 to 2033 |
| Base Year | 2025 |
| Forecast Period | 2026-2033 |
| Historical Data | 2022-2024 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product Type (Devices, Accessories & Consumables) • By End User (Specialty Clinics and Hospitals) |
| Regional Analysis/Coverage | North America (US, Canada), Europe (Germany, France, UK, Italy, Spain, Poland, Russsia, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Australia,ASEAN Countries, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Mexico, Colombia Rest of Latin America) |
|
Company Profiles |
Ambu A/S, Cardinal Health, Koninklijke Philips N.V, Drägerwerk AG & Co. KGaA, Medtronic, GE Healthcare, BD, Vyaire, Natus Medical Incorporated, Utah Medical Products, Inc. |

