3D Cell Culture Market Size Analysis:
To get more information on 3D Cell Culture Market - Request Free Sample Report
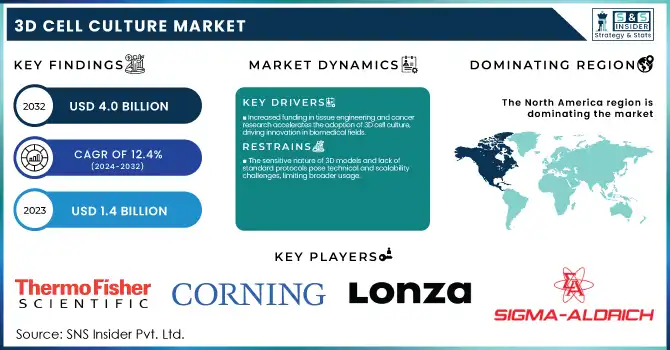
The 3D Cell Culture Market Size was valued at USD 1.4 Billion in 2023 and is expected to reach USD 4.0 billion by 2032, growing at a CAGR of 12.4% over the forecast period 2024-2032.
The 3D cell culture market is experiencing robust growth, driven by advancements in cell-based research and increasing governmental funding for biotechnology and regenerative medicine. According to the latest statistics from the U.S. National Institutes of Health (NIH), federal funding for stem cell research surpassed $2 billion in 2023, reflecting a 10% increase compared to 2022. Similarly, the European Commission allocated over €1.8 billion in 2023 under Horizon Europe’s health cluster, emphasizing regenerative medicine and tissue engineering. In parallel, the Indian Department of Biotechnology announced a 15% increase in funding, earmarking over ₹500 crore for tissue engineering projects in its 2023–24 fiscal budget.
Adding to this, the NIH also unveiled a new $100 million regenerative medicine initiative in early 2024, aimed at enhancing research infrastructure for 3D cell culture systems in disease modeling and therapeutic applications. The UK’s Medical Research Council (MRC) increased its funding by 12% in 2023, directing £200 million towards projects integrating 3D bioprinting and scaffold-based platforms. Japan’s AMED allocated $50 million in 2023 to foster innovation in organ-on-chip systems and scaffold technologies, aligning with the country’s ambitious plans for biotechnological advancements.
This influx of financial support accelerates the adoption of 3D cell culture technologies across academia and the biopharmaceutical industry. Scaffold-based platforms are extensively utilized in drug discovery, toxicology testing, and regenerative medicine applications, aligning with governmental priorities in public health innovation. As governments globally emphasize reducing dependency on animal models and improving the efficiency of drug discovery pipelines, scaffold-based systems remain at the forefront of 3D cell culture innovations.
Market Dynamics
Drivers
-
3D cell culture models better replicate in vivo conditions, improving drug discovery and disease modeling. Their precision supports personalized medicine through tailored diagnostics and high-throughput screening.
-
Advanced scaffolds and magnetic/microfluidic bioprinting enhance tissue modeling and enable scalable production of 3D cultures, fueling industry growth.
-
Increased funding in tissue engineering and cancer research accelerates the adoption of 3D cell culture, driving innovation in biomedical fields.
Introduction 3D cell culture models have revolutionized the field of drug discovery and personalized medicine in biomedical research. 3D cultures resemble in vivo cellular environments far better than traditional 2D models do and therefore provide better data for assessing the efficacy and toxicity of drugs. This ability improves preclinical studies substantially and diminishes the drug failure rate at the time of clinical trials. For instance, the National Center for Advancing Translational Sciences (NCATS) reported a 30% improvement in predicting drug responses using 3D organoid cultures compared to conventional methods.
Take personalized medicine where 3D cultures play a key role in patient-specific therapeutics. As an example, the researchers at the Hubrecht Institute use patient-derived organoids to screen cystic fibrosis drugs and obtain personalized treatments. It underlines how 3D models can accompany drugs and therapies to deliver optimum therapies for diseases, such as cancer and genetic disorders. Moreover, automated imaging technologies, such as high-content screening (HCS) systems combined with 3D cultures, have increasingly promoted large-scale screening. 3D models utilized for high-throughput drug screening in 2023 were proven to boost modeling accuracy by 40% over 2D cell cultures, which greatly improved the process of identifying effective compounds.
Restraints
-
The substantial costs of bioprinting systems and specialized culture media deter small and mid-sized labs from adopting 3D cell culture solutions.
-
The sensitive nature of 3D models and lack of standard protocols pose technical and scalability challenges, limiting broader usage.
The complexity of handling 3D cell cultures as compared to 2D cell cultures is one of the major restraints in the 3D cell culture market. In contrast, 3D systems which are much more sensitive to environmental fluctuations like oxygen, nutrient supply, and pH - require careful nurturing. In addition, it is difficult to reproduce results due to the lack of standardized protocols according to each type of 3D cell culture system, such as scaffold-based, hydrogel-based, or organoid cultures. Such discrepancies result in variation in the research results which complicates multi-lab studies as well as upsizing to an industrial scale. Further, it requires specialized technical expertise to handle the complex processes associated with 3D culture, an infrastructure that many labs, particularly smaller ones, may not possess. These factors collectively slow down the widespread adoption of 3D cell culture technologies despite their advantages.
Segment analysis
By Technology
The scaffold-based segment accounted for the largest revenue share of 46% in 2023, owing to its unique potential for recapitulating the native cellular microenvironment. It can be widely applied in more sophisticated applications of tissue engineering and drug testing. In large part, its use has been further prompt due to government assistance. In 2023, the U.S. Food and Drug Administration (FDA) spent $25 million improving methods like these that will rely heavily on 3D scaffold-based cultures as a means to minimize animal testing. Moreover, Japan’s Ministry of Health, Labour, and Welfare initiated a consortium to boost 3D bioprinting technologies, with scaffold-based strategies being essential. Scaffold materials vary widely in their composition from hydrogels to synthetic polymers, allowing broad applicability in translational oncology and personalized medicine. The aforementioned advantages along with factors like rising governmental initiatives towards the development of organ-on-a-chip systems further establish segmental dominance.
By Application
The global stem cell research & tissue engineering segment emerged as the market leader in 2023, accounting for 33% market share owing to the rise in global investments and governmental policies for generalizing regenerative therapies. In 2023, the NIH identified $850 million worth of stem cell research funding, representing a 12% gain. Similarly, the “Stem Cell and Translational Medicine Development Plan,” published by the China Ministry of Science and Technology also promised 5 billion RMB for projects including 3D cell culture platforms. The growing adoption of tissue-engineered constructs for clinical trials also drives growth in this segment. This year, for example, the European Medicines Agency (EMA) awarded several Phase III trials making use of 3D-printed tissue scaffolds to deliver cartilage defect therapies. This segment leads the market, owing to the combination of government support, immature technology, and received regulatory approvals.
By End-use
The biotechnology and pharmaceutical companies segment dominated the market, accounting for a revenue share of 47% in 2023, owing to the increasing preference of the biopharmaceutical and pharmaceutical companies towards 3D cell culture technologies in drug discovery and toxicology studies. The FDA’s 2023 Annual Report shows a remarkable change toward using 3D culture systems, with 65% of new drugs approved utilizing 3D cell culture data instead of 2D systems. In 2023, Canada also granted the Government the sum of CAD 75 million, for biopharmaceutical research, in support of advanced 3D, countries of human, cultures, and northern fission platforms. They are utilizing these systems for accelerating preclinical validation giving them a possibility to cut down costs and period as well as effectively improve drug efficacy and safety profile. Such a trend is along with increased regulatory scrutiny and worldwide efforts with anticipation of minimizing animal testing, thus strengthening the leadership for the segment.
Regional Insights
The 3D cell culture market is dominated by the North American region with a 37% revenue share in 2023 and is driven by strong investment in research and development (R&D) and a well-established biopharmaceutical infrastructure, along with supportive government policy. A $1.2 billion funding spree for regenerative medicine at the National Institutes of Health (NIH) shows the U.S. has its eyes on the prize of stem cell and regenerative therapy efforts. Furthermore, Canada's early move into regenerative medicine includes its "Regenerative Medicine and Cell Therapy Strategy", which has received an initial injection of CAD 150 million. Further backing this commitment, Canada allocated over $20 million in 2020 for advancing stem cell research through programs like the Stem Cell Network's funding program and Genome Canada's genomics initiatives.
On the other hand, the 3D cell culture market in Asia-Pacific is projected to grow at the highest CAGR over the forecast period. This rapid growth can be attributed to a rise in government investments in biotechnology in countries such as China, Japan, and India. For example, in 2023, China experienced a 20% increase in research grants within the field of tissue engineering. India's biotechnology sector, spurred by the "Make in India" movement, inspired an 18 % year-on-year growth. These efforts are positioning the Asia-Pacific region as a major growth engine for the 3D cell culture market.

Get Customized Report as per Your Business Requirement - Enquiry Now
Recent Developments
-
In October 2024, Univercells Technologies introduced the Scale-X Nexo bioreactor, aimed at enhancing the efficiency of cell culture process development for various therapeutic applications.
-
In September 2023, Curi Bio launched two platforms, Nautilus and Stringray, designed to assist researchers in investigating 2D and 3D cell cultures, particularly in electrophysiology.
-
In July 2023, Canadian company 3D BioFibR secured an investment of USD 3.52 million to expand its facility and introduce collagen fiber products for 3D bioprinting.
-
In July 2023, Vernal Biosciences and REPROCELL Inc. have partnered to supply mRNA services at scale for clinical and research purposes in Japan. This approach fits with REPROCELL's goal to market cutting-edge preclinical & clinical research solutions.
Key Players
Key Service Providers/Manufacturers
-
Thermo Fisher Scientific (Thermo Scientific™ 3D Cell Culture, Gibco™ 3D Culture System)
-
Corning Incorporated (Corning® Matrigel® Matrix, Corning® Elplasia® Microplates)
-
Lonza Group (PureCol® Collagen, MatriGel™ Basement Membrane Matrix)
-
Sigma-Aldrich (Merck) (Sigma 3D Culture Reagents, Ultra-low Attachment Surface Plates)
-
3D Biotek LLC (3D Cell Culture Kits, 3D Culture Plates)
-
ReproCELL (CELLARTIS® 3D Human Hepatocytes, ReproLiver™)
-
CELLINK (CELLINK Bioink, INKredible™ Bioprinter)
-
KURABO INDUSTRIES LTD. (Kurabo 3D Cell Culture Matrix, Kurabo Scaffold)
-
InSphero AG (InSphero 3D Microtissues, 3D InSight™)
-
Becton Dickinson (BD Biosciences) (BD Matrigel™, BD Falcon™ 3D Cell Culture Plates)
Key Users
-
Pfizer
-
Johnson & Johnson
-
Roche
-
Novartis
-
Bristol-Myers Squibb
-
AstraZeneca
-
Merck & Co.
-
Sanofi
-
Eli Lilly and Company
-
AbbVie
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 1.4 Billion |
| Market Size by 2032 | USD 4.0 Billion |
| CAGR | CAGR of 12.4% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Technology (Scaffold Based {Hydrogels, Polymeric Scaffolds, Micropatterned Surface Microplates, Nanofiber Base Scaffolds}, Scaffold Free {Hanging Drop Microplate, Spheroid Microplates with ULA Coating, Magnetic Levitation}, Bioreactors, Microfluidics, Bioprinting) • By End-use (Biotechnology and Pharmaceutical Companies, Academic & Research Institutes, Hospitals, Others) • By Application (Cancer Research, Stem Cell Research & Tissue Engineering, Drug Development & Toxicity Testing, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Thermo Fisher Scientific, Corning Incorporated, Lonza Group, Sigma-Aldrich (Merck), 3D Biotek LLC, ReproCELL, CELLINK, KURABO INDUSTRIES LTD., InSphero AG, Becton Dickinson (BD Biosciences) |
| Key Drivers | • 3D cell culture models better replicate in vivo conditions, improving drug discovery and disease modeling. Their precision supports personalized medicine through tailored diagnostics and high-throughput screening. • Advanced scaffolds and magnetic/microfluidic bioprinting enhance tissue modeling and enable scalable production of 3D cultures, fueling industry growth. |
| Restraints | • The substantial costs of bioprinting systems and specialized culture media deter small and mid-sized labs from adopting 3D cell culture solutions. • The sensitive nature of 3D models and lack of standard protocols pose technical and scalability challenges, limiting broader usage. |

