Anesthesia Monitoring Devices Market Report Scope & Overview:
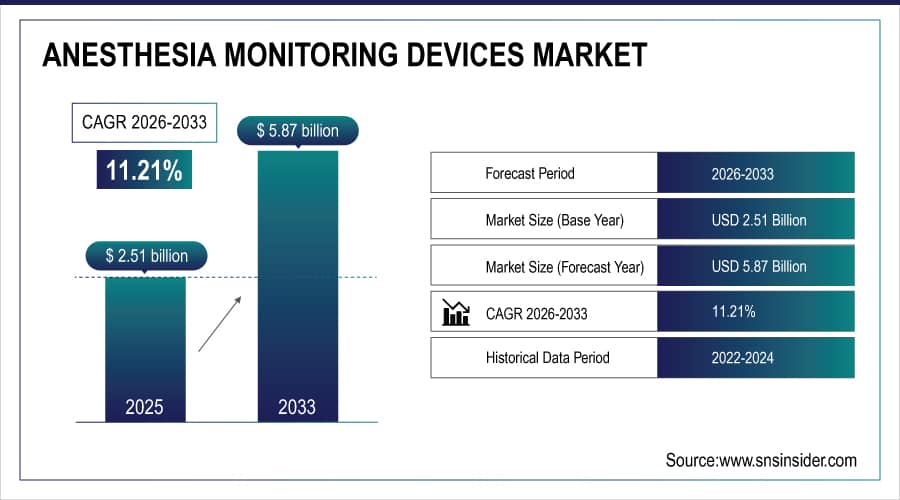
The Anesthesia Monitoring Devices Market size is estimated at USD 2.51 billion in 2025 and is expected to reach USD 5.87 billion by 2033, growing at a CAGR of 11.21% over the forecast period of 2026-2033.
Growing emphasis on patient safety during anesthetic administration, the prevalence of chronic disorders requiring surgical intervention, and the increase in surgical operations globally are driving the global anesthesia monitoring devices market trend. The adoption of minimally invasive operations, the integration of AI and machine learning for real-time patient data analysis, and advanced monitoring technologies are all having a big impact on market growth. The adoption of advanced anesthesia monitoring systems in hospitals and ambulatory surgery centers worldwide is being accelerated by increased investments in healthcare infrastructure, especially in emerging economies, and strict regulatory requirements for patient monitoring during surgical procedures.
For instance, in April 2024, the implementation of enhanced anesthesia safety protocols led to a 22% increase in advanced monitoring device adoption across major surgical centers in North America, improving patient outcomes and reducing perioperative complications.
Anesthesia Monitoring Devices Market Size and Forecast:
-
Market Size in 2025E: USD 2.51 billion
-
Market Size by 2033: USD 5.87 billion
-
CAGR: 11.21% from 2026 to 2033
-
Base Year: 2025
-
Forecast Period: 2026–2033
-
Historical Data: 2022–2024
To Get More Information On Anesthesia Monitoring Devices Market - Request Free Sample Report
Anesthesia Monitoring Devices Market Report Highlights
-
Rising number of surgical procedures globally, including cardiac, orthopedic, and emergency surgeries, driving demand for reliable anesthesia monitoring systems.
-
Integration of AI-powered analytics, wireless connectivity, and cloud-based data management for enhanced patient monitoring and decision-making support.
-
Development of portable and compact monitoring devices enabling use in diverse clinical settings, including ambulatory surgery centers and emergency departments.
-
Implementation of remote monitoring capabilities, telemedicine integration, and real-time alerts for anesthesiologists to improve patient safety and clinical efficiency.
-
Increasing focus on multi-parameter monitoring systems combining capnography, pulse oximetry, ECG, blood pressure, and temperature monitoring in single integrated platforms.
-
Strategic collaborations between medical device manufacturers, healthcare providers, and technology companies to develop next-generation anesthesia monitoring solutions.
-
FDA, CE Mark, and regulatory bodies worldwide establishing stringent safety standards, promoting clinical validation studies, and encouraging adoption of evidence-based monitoring practices.
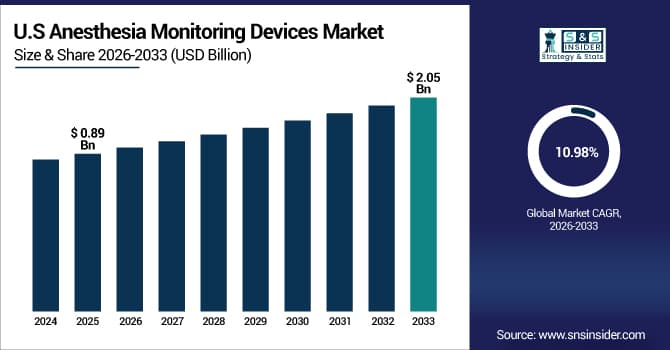
The U.S. Anesthesia Monitoring Devices Market is estimated at USD 0.89 billion in 2025 and is expected to reach USD 2.05 billion by 2033, growing at a CAGR of 10.98% from 2026-2033.
Due to its sophisticated healthcare system, huge surgery volume, and strict patient safety laws, the U.S. has the biggest market share. Widespread use of integrated anesthesia workstations, rising rates of surgically necessary chronic illnesses, and significant healthcare spending all contribute to market expansion. Additionally, the FDA's regulatory backing, the early adoption of cutting-edge monitoring technology, and the presence of top medical device makers establish the United States as the world's leading regional market.
Anesthesia Monitoring Devices Market Growth Drivers:
-
Technological Advancement in Monitoring Systems is Driving the Anesthesia Monitoring Devices Market Growth
The growth of the anesthetic monitoring devices market is primarily driven by technological innovation, which is fueled by the integration of sophisticated sensors, artificial intelligence algorithms, and wireless connectivity that allow for the transmission and analysis of patient data in real time. These developments improve patient safety during surgical procedures, decrease human error, and improve clinical decision-making. The creation of automated alarm systems and multi-parameter monitoring systems with user-friendly interfaces is increasing market penetration in ambulatory surgery centers and hospitals, which is boosting global market expansion.
For instance, in August 2024, AI-integrated anesthesia monitoring systems accounted for approximately 26% of new installations in U.S. hospitals, reflecting growing demand for intelligent monitoring solutions and expanding market share in advanced surgical facilities.
Anesthesia Monitoring Devices Market Restraints:
-
High Equipment Costs and Maintenance Requirements are Hampering the Anesthesia Monitoring Devices Market Growth
The market growth for anesthesia monitoring devices is severely constrained by high equipment costs and continuous maintenance needs, which have an especially negative impact on adoption in emerging economies and healthcare facilities with tight budgets. The operation of sophisticated integrated anesthetic workstations and monitoring systems necessitates a large initial investment, frequent calibration, software upgrades, and skilled staff. These financial obstacles affect market expansion in emerging nations where healthcare budgets are still tight, restrict market penetration in environments with limited resources, and postpone equipment replacement cycles.
Anesthesia Monitoring Devices Market Opportunities:
-
Expansion in Ambulatory Surgery Centers Drive Future Growth Opportunities for the Anesthesia Monitoring Devices Market
Due to cost-effectiveness, shorter hospital stays, and patient desire for same-day surgeries, outpatient surgical procedures are growing at a rapid rate, which presents an opportunity for the market for anesthetic monitoring devices in ambulatory surgery centers. Compact, portable, and affordable anesthetic monitoring systems made especially for ambulatory settings are in high demand as a result of this trend. Manufacturers can take advantage of this expanding market segment, broaden their market reach, and generate substantial revenue growth in the upcoming years by integrating electronic health records, improving workflow efficiency, and improving monitoring capabilities.
For instance, in May 2024, the American Society of Anesthesiologists reported that 68% of surgical procedures in the U.S. were performed in outpatient settings, highlighting the growing demand for specialized anesthesia monitoring equipment in ambulatory surgery centers.
Anesthesia Monitoring Devices Market Segment Analysis
-
By product, integrated anesthesia workstation held the largest share of around 46.82% in 2025E, and the advanced anesthesia monitor segment is expected to register the highest growth with a CAGR of 11.89%.
-
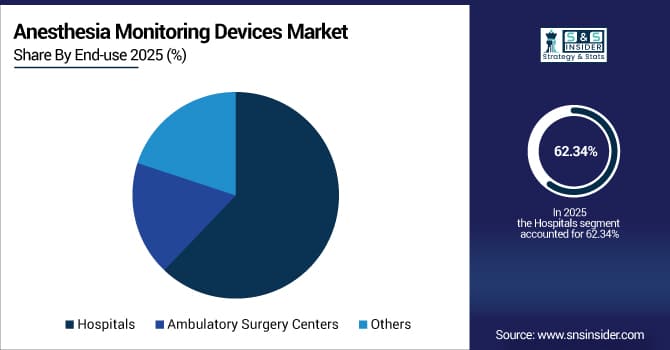
By end-use, hospitals dominated the market with approximately 62.34% share in 2025E, while ambulatory surgery centers are expected to register the highest growth with a CAGR of 12.15%.
By Product, Integrated Anesthesia Workstation Leads the Market, While Advanced Anesthesia Monitor Registers Fastest Growth
The integrated anesthesia workstation segment accounted for the highest revenue share of approximately 46.82% in 2025, owing to comprehensive monitoring capabilities, space efficiency, and seamless integration of ventilation and monitoring functions in a single platform.
The advanced anesthesia monitor segment is anticipated to achieve the highest CAGR of nearly 11.89% during the 2026–2033 period, driven by rising demand for specialized monitoring capabilities including depth of anesthesia monitoring, advanced hemodynamic parameters, and cerebral function monitoring.
By End-use, Hospitals Dominate, While Ambulatory Surgery Centers Show Rapid Growth
The hospitals segment held the largest revenue share of approximately 62.34% in 2025, owing to high volume of complex surgical procedures, availability of comprehensive surgical infrastructure, and regulatory requirements for advanced patient monitoring in inpatient settings.
The ambulatory surgery centers segment is predicted to grow at the strongest CAGR of approximately 12.15% during 2026–2033, driven by rapid expansion of outpatient surgical facilities, cost-containment pressures favoring outpatient procedures, and technological advancements enabling safe anesthesia delivery in non-hospital settings.
Anesthesia Monitoring Devices Market Regional Highlights:
Asia Pacific Anesthesia Monitoring Devices Market Insights:
Asia Pacific is the fastest-growing region in the anesthesia monitoring devices market, expanding at a CAGR of 12.67% during the forecast period. The region’s expansion is driven by rapidly expanding healthcare infrastructure, increasing surgical volumes, and rising healthcare expenditure in emerging economies including China, India, and Southeast Asian nations. Contributing factors include growing medical tourism industry, increasing prevalence of chronic diseases requiring surgical treatment, and government initiatives to improve healthcare access and quality. Expansion of private hospital networks, establishment of specialty surgical centers, and increasing adoption of advanced medical technologies are stimulating market growth. Rising awareness of patient safety standards, growing middle-class population with increased healthcare spending capacity, and favorable regulatory environments supporting medical device imports drive growth in the Asia Pacific region.
Get Customized Report as Per Your Business Requirement - Enquiry Now
North America Anesthesia Monitoring Devices Market Insights:
The anesthesia monitoring devices market in North America held the largest share of revenue of nearly 38.45% in 2025, attributed to better developed healthcare infrastructure, larger number of surgical procedures performed, and much stringent regulations on patient safety requiring extensive monitoring of anesthesia process. Many factors contributing to its growth comprehend earlier acceptance of integrated anesthesia workstations, high incidence of chronic conditions which require surgical management, quickly accessible insurance coverage and acceptance of newest monitoring inputs. In addition, established presence of leading manufacturers of medical devices, a high rate of clinical research, enormous expenses on healthcare, and stringent FDA approval processes that guarantee high quality provide dominancy and a high number of sales of anesthesia monitoring devices in the global market.
Europe Anesthesia Monitoring Devices Market Insights:
Geographically, the Europe anesthesia monitoring devices market depicts the second-largest region after North America by virtue of strong healthcare systems in European countries along with high number of surgical procedures, and rigid regulatory framework ensuring safe anesthesia administration to patient. Key market growth drivers are increasing adoption of advanced monitoring technologies, growing geriatric population with rising surgical interventions, favorable reimbursement policies, and harmonized medical device regulation under Medical Device Regulation (MDR). Steady investments in healthcare modernization, wide presence of leading medical device companies, and focus on evidence-based clinical practices allows continued market growth in most of the major European countries.
Latin America (LATAM) and Middle East & Africa (MEA) Anesthesia Monitoring Devices Market Insights:
The growth of anesthesia monitoring devices market in Latin America and Middle East & Africa is driven by the growing healthcare infrastructure, rising surgical volumes and increasing awareness on patient safety standards during delivery of anesthesia. Market expansion is also driven by rising investments in hospital modernization, expanding private healthcare sector and government measures to improve quality of surgical care. Drivers and restraints: the rising uptake of basic and integrated anesthesia monitoring systems, medical device procurement programs, and international partnerships assist with penetration of the market and technology transfer. Continued urbanization, emerging middle-income classes, and improving access to healthcare in these regions drive the need for and usage of basic anesthesia monitors in these markets enabling further growth.
Anesthesia Monitoring Devices Market Competitive Landscape:
GE HealthCare (spun off from GE in 2023) is a global leader in medical technology, offering a comprehensive portfolio of anesthesia delivery and monitoring solutions, including the acclaimed Aisys and Avance integrated workstations.
-
In March 2025, GE HealthCare announced its new integrated platform of "Carestation" which has embedded AI analytics capabilities for predictive ventilation and hemodynamic management that can support clinician decision making.
Drägerwerk AG & Co. KGaA (est. 1889) is a German leader in medical and safety technology, renowned for its high-performance anesthesia workstations (Perseus and Atlan) and advanced patient monitoring systems used in critical care environments worldwide.
-
In November 2024, Dräger wins FDA 510(k) clearance for its new mini-anesthesia monitor for ambulatory surgery centers, targeting the low hanging fruit of the faster growing outpatient segment
Medtronic plc (est. 1949) is a global healthcare solutions giant with a strong presence in patient monitoring through its Patient Monitoring and Respiratory Interventions businesses, offering a range of monitors and consumables for anesthesia management.
-
In January 2025, Medtronic extended its agreement with a large health system to incorporate patient monitoring data, such as anesthesia measures into a single, enterprise-wide clinical data platform to improve care coordination.
Anesthesia Monitoring Devices Market Key Players:
-
GE HealthCare
-
Philips Healthcare
-
Drägerwerk AG & Co. KGaA
-
Medtronic plc
-
Masimo Corporation
-
Nihon Kohden Corporation
-
Mindray Medical International Limited
-
Spacelabs Healthcare (OSI Systems)
-
Schiller AG
-
Becton, Dickinson and Company (BD)
-
Getinge AB
-
Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
-
Fukuda Denshi
-
Criticare Systems, Inc.
-
Smiths Medical (ICU Medical)
-
Heyer Medical AG
-
Infinium Medical
-
Chirana Medical
-
EDAN Instruments
| Report Attributes | Details |
|---|---|
| Market Size in 2025 | USD 2.51 Billion |
| Market Size by 2033 | USD 5.87 Billion |
| CAGR | CAGR of 11.21% From 2026 to 2033 |
| Base Year | 2025 |
| Forecast Period | 2026-2033 |
| Historical Data | 2022-2024 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product (Basic Anesthesia Monitor, Integrated Anesthesia Workstation, Advanced Anesthesia Monitor) • By End-use (Hospitals, Ambulatory Surgery Centers, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | GE HealthCare, Philips Healthcare, Drägerwerk AG & Co. KGaA, Medtronic plc, Masimo Corporation, Nihon Kohden Corporation, Mindray Medical International Limited, Spacelabs Healthcare (OSI Systems), Schiller AG, Becton, Dickinson and Company (BD), Getinge AB, Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Fukuda Denshi, Criticare Systems, Inc., Smiths Medical (ICU Medical), Heyer Medical AG, Infinium Medical, Chirana Medical, EDAN Instruments |

