Biopharmaceutical CMO Market Size & Growth:
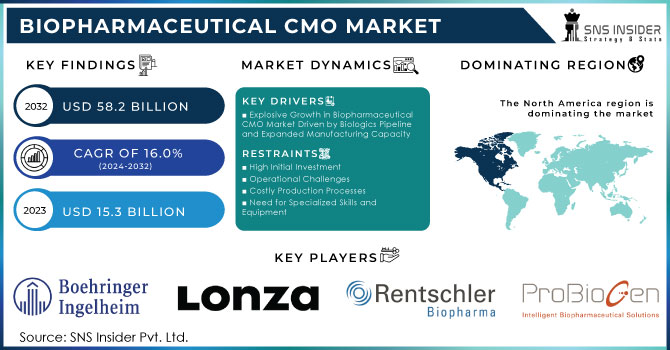
The Biopharmaceutical CMO Market size was valued at USD 15.3 billion in 2023 and is expected to reach USD 58.2 billion by 2032 with a growing CAGR of 16.0% over the forecast period of 2024-2032. The rising demand for outsourced services such as microbial fermentation and mammalian cell culture has been critical in driving the growth of the biopharmaceutical contract manufacturing organization (CMO) market. Over the last few years, there has been an increased tendency among biopharmaceutical companies to outsource such processes to contract manufacturers primarily due to the increasing focus on the development of biologics, including recombinant proteins and monoclonal antibodies. A significant increase in developing biologics has led to an upsurge in demand for outsourcing services, which is translated directly into a need for mammalian cell culture services from CMOs.
To Get More Information on Biopharmaceutical CMO Market - Request Sample Report
Outsourced animal cell culture services have grown by a stunning 10% since 2019, and mammalian cell culture capacity needs grew by 30%, according to Contract Pharma. The speed of growth accelerated from the widespread impact of the COVID-19 pandemic in 2020, which gave biopharmaceuticals an unprecedented boost. Increased investments from leading players in the biopharma sector to enhance productivity and efficiency have also increased outsourcing. More and more biopharma companies now outsource these more capital- and resource-intensive steps or indeed biomanufacturing processes in their entirety to improve operational efficiency and the capital costs of the given process.
The rising demand is forcing CMOs to increase their manufacturing volumes. In one such move, Cambrex, a U.S.-headquartered CDMO, has recently announced plans to expand its biopharmaceutical testing services at its U.S. facility. During the year 2022 itself, it added 11 cGMP laboratories at its U.S. facility, reflecting the rising scale of need for quite advanced technologies, including mass spectrometry, qPCR, and NGS, in the biopharma industry.
Some of the changing facets that characterize CMOs' selection of business models and strategies include that they are becoming increasingly reliant on single-use systems, which allows for faster production turnarounds and reduced operational costs. As much as there have been gigantic strides in these latest technological innovations, contract negotiations between CMOs and their clients are complex, which is majorly due to issues around regulatory compliance, intellectual property rights, and service pricing. As the biopharmaceutical industry continues to change, CMOs keep adjusting their business models to place them in favorable positions relative to the rising demands and the intricacies of the market in which they now compete.
Major Biopharma CMO Service Providers, By Region
| Region | Company Name | Description |
|---|---|---|
|
North America |
Catalent |
Offers drug delivery solutions and manufacturing. |
|
Lonza |
Specializes in biologics and pharmaceuticals. |
|
|
Patheon |
Provides a wide range of manufacturing services. |
|
|
AMRI |
Focuses on drug development and manufacturing. |
|
|
Recipharm |
Offers contract manufacturing for pharmaceuticals. |
|
|
Europe |
WuXi AppTec |
Provides integrated services in drug development. |
|
Boehringer Ingelheim |
Known for biopharmaceutical manufacturing services. |
|
|
Rentschler Biotechnologie |
Offers development and manufacturing of biopharmaceuticals. |
|
|
Famar |
Provides services for pharmaceuticals and cosmetics. |
|
|
Vetter Pharma |
Focuses on aseptic filling and packaging services. |
|
|
Asia-Pacific |
Samsung Biologics |
Offers end-to-end biopharmaceutical contract services. |
|
Fujifilm Diosynth Biotechnologies |
Specializes in mammalian cell culture and microbial fermentation. |
|
|
Jubilant Biosys |
Provides integrated drug discovery and development services. |
|
|
Sequirus |
Offers CMO services with a focus on vaccines. |
|
|
BioFactor |
Specializes in biopharmaceutical contract manufacturing. |
|
|
Latin America |
Instituto Biológico Argentino |
Provides CMO services in vaccine and biopharmaceutical manufacturing. |
|
Biogenesis Bago |
Focuses on biopharmaceutical manufacturing. |
|
|
Middle East |
Julphar |
Offers pharmaceutical and biopharmaceutical manufacturing services. |
|
Medochemie |
Provides a range of CMO services across various therapeutic areas. |
Biopharmaceutical CMO Market Dynamics
Drivers
-
Explosive Growth in Biopharmaceutical CMO Market Driven by Biologics Pipeline and Expanded Manufacturing Capacity
The biopharmaceutical contract manufacturing market is likely to grow explosively since pharmaceutical and biotechnology companies continue to accelerate their investments in outsourcing services. Lastly, an excellent pipeline of biologics with several products lined up for launch during the forecast period propels this market forward. With the rising completion of the development process of biopharmaceutical products, demand for outsourced manufacturing services will surge sharply. The growing number of drugs that get approval also contributes to market growth. For example, in 2023, 55 new drugs and biological products received U.S. FDA approval: with growth moving in this direction, the sector is gaining increased momentum this way.
Biopharmaceutical CMO collaborations and acquisitions are also fueling the market. About 30 percent of recombinant commercial products are produced by CMOs; however, this is restricted to some of the largest vendors in the industry. The U.S. remains one of the key geographies for outsourcing. Among the industry respondents, 30.1 percent of those respondents said they would prefer contract manufacturing at U.S. facilities within five years.
Another push factor is the increase in the capacity of the production of biologics undertaken by several CMOs. This number will also increase as more novel drugs enter clinical pipelines. The mammalian biomanufacturing capacity is increasing at the rate of 11.5% per year. Samsung Biologics is one of the companies investing millions of dollars to meet the growing demand, for example, the USD 2 billion manufacturing facility. These trends will further advance the biopharmaceutical CMO market as more companies outsource biologics.
Restraints
-
High Initial Investment
-
Operational Challenges
-
Costly Production Processes
-
Need for Specialized Skills and Equipment
Biopharmaceutical CMO Market Segmentation Analysis
By Source
The mammalian segment remained the market leader in 2023, with a share of over 56.9%, mainly due to the lack of in-house skills in the industry. The success can be attributed to the fact that mammalian segment technology is the only one that can match human-like post-translational modifications for complex protein therapeutics. Novel and enhanced expression systems, improved process monitoring solutions, cell line engineering tools, automated screening techniques, and disposable devices have benefited mammalian segments highly.
On the other hand, the non-mammalian segment is expected to grow very rapidly with a CAGR of 7.7% during the forecast period. It is these microbial cell lines which are very efficient in terms of their production platforms that have been causing the growth of the non-mammalian segment. Innovative approaches have taken place for exploring the potential of different microbes, thereby contributing to the growth of non-mammalian biopharmaceutical manufacturing. Its use for the production of large-volume biopharmaceutical products is also increasing the revenue of this segment.
By Services
The contract manufacturing segment accounted for the largest share of the market in 2023 with more than 58.0%. Strong growth in this segment is due to the growing trend of biopharmaceutical companies outsourcing their research and development activities. A large number of CMOs, including opportunistic players, offer end-to-end services from cell cultivation to fill/finish operations, which make up for total care to biopharma companies. Companies are heavily investing to outsource the manufacturing component of their product development programs. Thus, this segment is bound to grab a considerable market share.
Contract research will witness the highest growth, at a CAGR of 6.4% in the biopharmaceutical CMO and CRO market. Contract research organizations position themselves to capture emerging opportunities within the industry. New entrants into the market, as well as small-scale biopharmas, focused on discovering new biopharmaceutical products, are expected to increasingly utilize contract research services to support their discovery and development programs, thereby driving segmental growth. Moreover, severe regulations imposed on the approval process of biopharmaceuticals will further boost this segment's growth.
By Product
The Biologics segment remained the market leader in 2023, with more than a 79.9% revenue share. This can be attributed to biologics' high specificity, complex production processes, and higher success rate than traditional drug molecules. Advanced technologies, such as one-time bioreactors, continuous purification processing, disposable plastic containers, and real-time quality analysis enable CMOs to cater to the increasing demand for biologics production efficiently. In addition, outsourced budgets spent on the development of biologics denote a large market share of the segment. The expanded market for biologics, as referred to earlier, is putting an even greater onus upon developers and regulators to catch up. As such, it is only working to accelerate the uptake of CMO services and, consequently, this segment.
The segment of biosimilars is likely to reach the fastest growth rate at a CAGR of 8.7% during the forecast period. Several companies are investing in biosimilar development to exceed the safety, efficacy, disposition, or cost of earlier in-class innovator drugs. Given this increasing competition among innovator manufacturers, CMOs are expected to benefit owing to their indispensable support in the development and production of biosimilars.
Biopharmaceutical CMO Market Regional Outlook
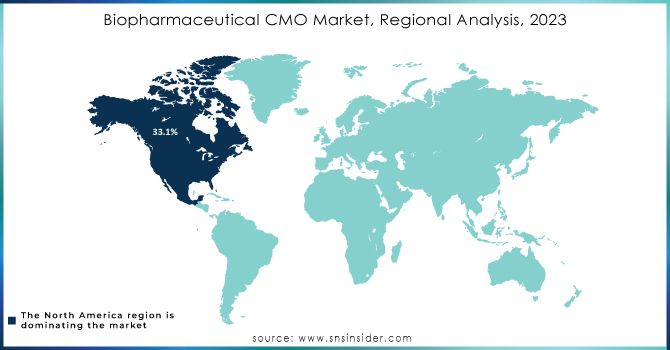
North America dominated the market in 2023, producing the highest revenue, at around 33.1% of the biopharmaceutical CMO market. It's because North America is predominant in terms of the strong local presence of several service providers and a large number of the CMOs that manufacture approved products for the U.S. Small- and mid-sized biopharmaceutical companies in the United States face challenges to set up properly equipped manufacturing facilities with limited resources and budgets. Instead, they mainly use CMOs. Thus, the interaction of CMOs and SMEs has led to a strengthening of the US market leadership. The US-based CMOs and CROs increasingly invest in advanced research and manufacturing technologies, and higher penetration of these facilities is propelling market growth. Also, the quality of services that are provided is opening enough a much-needed link between biopharma companies and CMOs in the country.
Asia Pacific is expected to grow considerably and emerge as the fastest-growing market for biopharmaceutical R&D and manufacturing, in the course of the forecast period. Such growth can be ascribed to the factors relating to better infrastructural facilities, a huge available pool of study subjects, and changes in regulations. The increasing R&D costs in the U.S. have led to the increased interest of many firms in biopharma to take up product development in Asian countries, thus broadening the scope of growth for the region. China, especially, consumed a substantial market share in 2023 with the development of rising R&D activities and also with the increased trend of outsourcing operations to local market players by overseas biopharmaceutical firms. This outsourcing enables the global innovators to restructure the efforts on R&D with low cost. Also, the Chinese government provides good funding for biotechnology, and market benefits from the expert professionals returning from the U.S. and Europe, increasing the competence of the domestic CROs and CMOs.
Do You Need any Customization Research on Biopharmaceutical CMO Market - Enquire Now
Biopharmaceutical CMO Companies
-
Boehringer Ingelheim GmbH
-
Lonza Group AG
-
Inno Biologics Sdn Bhd
-
Rentschler Biopharma SE
-
Biomeva GmbH
-
ProBioGen AG
-
Samsung Biologics
-
Recipharm AB
-
Fujifilm Diosynth Biotechnologies U.S.A., Inc.
-
Toyobo Co., Ltd.
-
Samsung Biologics
-
Thermo Fisher Scientific Inc (Patheon & PPD)
-
CMC Biologics
-
WuXi Biologics
-
AbbVie Inc.
-
Binex Co., Ltd.
-
Charles River Laboratories International, Inc.
-
ICON Plc
-
Parexel International Corporation
-
Laboratory Corporation of America Holdings
-
Siegfried Holding AG
-
Cambrex Corporation
-
Catalent, Inc., and others.
Recent Developments
-
In March 2024, Lonza announced an agreement to purchase Roche's big-batch biologics manufacturing facility in Vacaville, U.S., from F. Hoffmann-La Roche Ltd (Roche).
-
In October 2023, Vaxcyte, Inc., a U.S.-based biotechnology firm that is involved in the development of cutting-edge vaccines, announced an expansion to its collaboration with Lonza. This partnership will support the global commercial manufacturing of Vaxcyte's broad-spectrum pneumococcal conjugate vaccines (PCVs).
| Report Attributes | Details |
| Market Size in 2023 | US$ 15.3 Billion |
| Market Size by 2032 | US$ 58.2 Billion |
| CAGR | CAGR of 16.0% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Source Outlook (Mammalian, Non-Mammalian) • By Service Outlook (Contract Manufacturing, Contact Research) • By Product outlook (Biologics, Biosimilars) |
| Regional Analysis/Coverage | North America (USA, Canada, Mexico), Europe (Germany, UK, France, Italy, Spain, Netherlands, Rest of Europe), Asia-Pacific (Japan, South Korea, China, India, Australia, Rest of Asia-Pacific), The Middle East & Africa (Israel, UAE, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Rest of Latin America) |
| Company Profiles | Boehringer Ingelheim GmbH, Lonza Group AG, Inno Biologics Sdn Bhd, Rentschler Biopharma SE, JRS Pharma, Biomeva GmbH, ProBioGen AG, Samsung Biologics , Recipharm AB , WuXi Biologics , Fujifilm Diosynth Biotechnologies U.S.A., Inc., Toyobo Co., Ltd., Samsung Biologics, Thermo Fisher Scientific Inc, CMC Biologics and Others |
| Key Drivers | • Explosive Growth in Biopharmaceutical CMO Market Driven by Biologics Pipeline and Expanded Manufacturing Capacity |
| Market Restraints | • High Initial Investment • Operational Challenges • Costly Production Processes • Need for Specialized Skills and Equipment |

