Drug Eluting Stent Market Report Scope & Overview:
The Drug Eluting Stent Market size was estimated at USD 7.80 Billion in 2023 and is expected to reach USD 14.98 Billion by 2031 at a CAGR of 8.5% during the forecast period of 2024-2031.
Drug-eluting stents are tiny expandable mesh tubes constructed of medical-grade stainless steel or cobalt alloy metal that are placed into atherosclerotic patients' coronary arteries. The stent assists in keeping the artery open in order to deliver a medication that prevents arterial blockage and promotes blood flow in the artery.
Get More Information on Drug eluting stents Market - Request Sample Report
Stents are most commonly utilised in operations like coronary angioplasty. Drug-eluting stents are used to treat coronary artery disease and coronary angioplasty procedures such as percutaneous coronary interventions (PCI) and others. medication eluting stents are made up of three parts: the stent platform, the polymer coating (which keeps the medication inside the stent), and the drug.
MARKET DYNAMICS
DRIVERS
-
Over the projected period, increasing product approvals are likely to fuel the worldwide drug eluting stents market growth.
-
The rising frequency of cardiovascular illnesses will increase demand for therapy and surgical intervention.
RESTRAIN
-
Regulatory issues and the high price of DES Alternative therapies poses a threat.
The introduction of new DES products to the market might be slowed by stringent regulatory regulations and approval processes, impeding their commercialization. Drug-eluting stents are often more expensive than bare-metal stents, making them less accessible to patients in areas with poor healthcare resources.
OPPORTUNITY
-
Personalised medicine is a growing sector in emerging economies.
CHALLENGES
-
Concerns about long-term safety expirations of patents.
Despite substantial advancements, there may still be questions regarding the long-term safety of DES and the possibility for adverse consequences such as stent thrombosis and restenosis. Patent expirations for some drug-eluting stent technology may result in greater competition and price erosion for existing players.
IMPACT OF RUSSIA-UKRAINE WAR
The report's study of the impact of the Russia-Ukraine War on the sector is one of its most prominent features. The violence has clearly had an impact on the market, and the research examines how this has occurred. The study gives significant information to individuals wishing to invest in the business by offering a nuanced view on how the Drug-Eluting stent market has been shaped by both war and pandemic. Despite the difficulties that the Drug-Eluting Stents industry has endured, the research is upbeat about its prospects. It forecasts that the market will transform in the next few years, responding to the new circumstances brought forth by the epidemic and conflict. The study gives vital insights into how the industry will change in the next few years by incorporating an analysis of the influence of these events on the market.
To summarise, the Drug-Eluting Stents industry has clearly encountered considerable hurdles in recent years. However, the research offers promise for the future, offering a picture of an industry that will adapt and grow in response to changing conditions.
IMPACT OF ONGOING RECESSION
Economic recessions can have an impact on healthcare spending at the institutional and governmental levels. Hospitals and healthcare professionals may suffer funding cuts or resource limits, which might result in more cautious usage of medical devices such as drug-eluting stents. Product Mix Shift The impact of a recession may cause a shift in the product mix within the DES market. Patients and healthcare providers may prioritise cost-effective treatments, potentially increasing demand for bare-metal stents over drug-eluting stents. Reduced R&D Investment During a recession, medical device makers may encounter financial issues, resulting in lower R&D spending. This might have an influence on the market release of new and innovative DES products, impeding technical developments.
KEY MARKET SEGMENTATION
By Type
-
Coronary Stenting
-
Peripheral Stenting
By Scaffold
-
Cobalt-Chromium
-
Platinum-Chromium
-
Nitinol
-
Others
By Drug
-
Sirolimus
-
Paclitaxel
-
Zotarolimus
-
Everolimus
-
Others
By End User
-
Hospitals
-
Specialty Clinics
REGIONAL COVERAGE
North America
-
US
-
Canada
-
Mexico
Europe
-
Eastern Europe
-
Poland
-
Romania
-
Hungary
-
Turkey
-
Rest of Eastern Europe
-
-
Western Europe
-
Germany
-
France
-
UK
-
Italy
-
Spain
-
Netherlands
-
Switzerland
-
Austria
-
Rest of Western Europe
-
Asia Pacific
-
China
-
India
-
Japan
-
South Korea
-
Vietnam
-
Singapore
-
Australia
-
Rest of Asia Pacific
Middle East & Africa
-
Middle East
-
UAE
-
Egypt
-
Saudi Arabia
-
Qatar
-
Rest of Middle East
-
-
Africa
-
Nigeria
-
South Africa
-
Rest of Africa
-
Latin America
-
Brazil
-
Argentina
-
Colombia
-
Rest of Latin America
REGIONAL ANALYSES
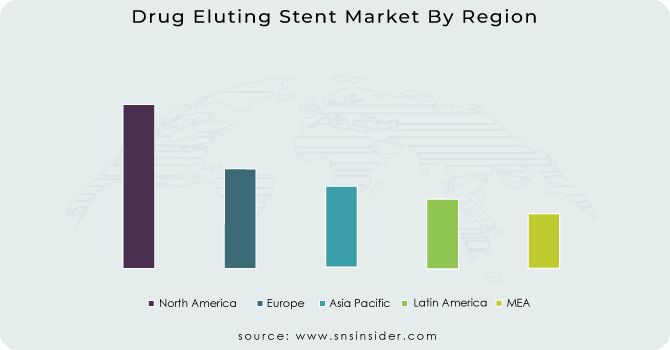
The North American market is expected to grow significantly during the forecast period due to the prevalence of a sizable patient population suffering from cardiovascular diseases, the availability of cutting-edge healthcare infrastructure, and the adoption of cutting-edge technologies. The United States is the country primarily responsible for the expansion of the North American DES market, with a number of big firms established there.
The European market for Drug Eluting Stents (DES) is expected to grow gradually throughout the forecast period. The region's expanding older population, as well as the increased prevalence of cardiovascular illnesses, are both driving market growth. The presence of a well-established healthcare infrastructure and a considerable number of healthcare professionals in the area are the primary drivers of the growth of the DES market in Europe. The rise of the DES market in Europe is also expected to be driven by an increase in the number of research initiatives and product launches by leading players in the field.
Get Customized Report as per Your Business Requirement - Request For Customized Report
Key Players
The major players are Abbott Laboratories, Boston Scientific Corporation, Medtronic, Cordis Corporation (a Johnson & Johnson company), Terumo Corporation, Biosensors International Group, Ltd, Biotronik SE & Co. KG, St. Jude Medical, Inc. and others.
Medtronic-Company Financial Analysis
RECENT DEVELOPMENTS
Food and Drug Administration: In September 2021, The US Food and Drug Administration has approved Biotronik's Orsiro Mission bioabsorbable polymer drug-eluting stent system (BP-DES). The gadget is believed to have an ultrathin strut design that is the thinnest available in the United States.
Abbott: In June 2021, gained FDA clearance for its XIENCE family of stents for one-month (for 28 days) DAPT labelling for high bleeding risk (HBR) patients in the United States. The CE mark has already been granted to the product.
| Report Attributes | Details |
| Market Size in 2023 | US$ 7.80 Bn |
| Market Size by 2031 | US$ 14.98 Bn |
| CAGR | CAGR of 8.5% From 2024 to 2031 |
| Base Year | 2023 |
| Forecast Period | 2024-2031 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Type (Coronary Stenting, Peripheral Stenting) • By Scaffold (Cobalt-Chromium, Platinum-Chromium, Nitinol, Others) • By Drug (Sirolimus, Paclitaxel, Zotarolimus, Everolimus, Others) • By End User (Hospitals, Specialty Clinics) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]). Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia Rest of Latin America) |
| Company Profiles | Abbott Laboratories, Boston Scientific Corporation, Medtronic, Cordis Corporation (a Johnson & Johnson company), Terumo Corporation, Biosensors International Group, Ltd, Biotronik SE & Co. KG, St. Jude Medical, Inc. |
| Key Drivers | • Over the projected period, increasing product approvals are likely to fuel the worldwide drug eluting stents market growth. • The rising frequency of cardiovascular illnesses will increase demand for therapy and surgical intervention. |
| Market Restraints | • Regulatory issues and the high price of DES Alternative therapies poses a threat. |

