Medical Device Cleaning Market Size & Trends:
Get More Information on the Medical Device Cleaning Market - Request Sample Report
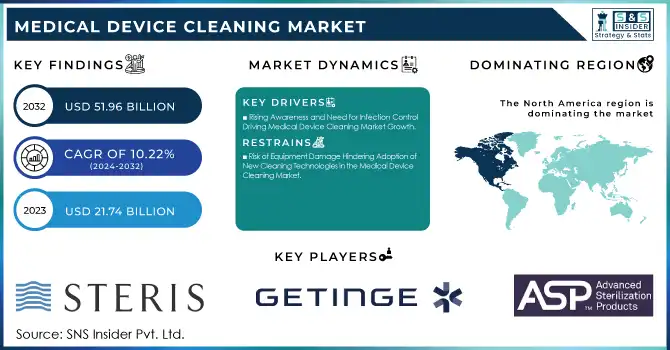
The Medical Device Cleaning Market size was valued at USD 21.74 billion in 2023 and is expected to reach USD 51.96 billion by 2032, growing at a CAGR of 10.22% from 2024-2032.
The medical device cleaning market has experienced significant growth driven by increasing awareness of the need for hygiene and infection prevention in healthcare settings. This growing emphasis on cleanliness has resulted in stricter government regulations and guidelines that demand advanced cleaning solutions for medical devices. Consequently, healthcare facilities are adopting more sophisticated cleaning technologies to comply with these regulations, further fueling market growth. As the number of medical procedures rises globally, with 234 million operations performed worldwide each year according to the World Health Organization, there is an escalating need for effective and reliable cleaning methods, which is directly boosting demand for cleaning devices and solutions.
The growing number of surgeries and diagnostic procedures is creating a heightened need for strict cleaning protocols to ensure patient safety and prevent cross-contamination. Hospitals and clinics are responding to this demand by investing in automated cleaning systems, sterilizers, and disinfectants that improve efficiency and reduce human error. These investments are essential for maintaining high standards of care, which, in turn, drives the demand for more advanced and efficient cleaning solutions. Technological innovations, such as the development of robotic cleaning systems and eco-friendly disinfectants, are also contributing to this growing demand by offering more sustainable and effective alternatives.
Looking ahead, the future of the medical device cleaning market appears promising, with significant opportunities emerging from technological advancements and evolving healthcare needs. The focus on automation and sustainability is expected to shape the next phase of growth, as healthcare facilities seek solutions that are both cost-effective and environmentally friendly. As the global healthcare sector expands and the focus on infection control intensifies, the demand for cutting-edge cleaning systems will continue to rise.
MARKET DYNAMICS
DRIVERS
-
Rising Awareness and Need for Infection Control Driving Medical Device Cleaning Market Growth
The increasing prevalence of hospital-acquired infections has significantly raised awareness about the critical role of infection control in healthcare settings. As HAIs pose a serious threat to patient safety and treatment outcomes, healthcare facilities are placing greater emphasis on stringent cleaning, disinfection, and sterilization procedures for medical devices. This growing focus on infection prevention is fueling demand for reliable and effective cleaning solutions, as hospitals, clinics, and surgical centers strive to minimize the risk of contamination. Stringent regulations and heightened public awareness further emphasize the need for clean medical devices, pushing healthcare providers to adopt advanced cleaning technologies and practices. This drive to enhance infection control is a major catalyst for the growth of the medical device cleaning market.
-
Technological Advancements Revolutionizing the Medical Device Cleaning Market
Innovative advancements in cleaning technologies are transforming the medical device cleaning market. The development of automated cleaning systems, advanced disinfectants, and smart devices has significantly improved cleaning effectiveness and efficiency. On October 29, 2024, Bolb, Inc. introduced the UVC LED S3535-H, delivering over 150 mW per chip for powerful disinfection at 265 nm, maintaining more than 70% output after 10,000 hours and withstanding temperatures up to 260°C. These innovations, such as automated systems with precise cleaning cycles and smart devices with real-time monitoring, ensure higher hygiene standards, reduce labor, and enhance patient safety. Moreover, novel disinfectants target a broader range of pathogens, advancing infection control and fueling demand for advanced cleaning solutions in healthcare.
RESTRAINTS
-
Risk of Equipment Damage Hindering Adoption of New Cleaning Technologies in the Medical Device Cleaning Market
Medical devices are often delicate and require precise handling during cleaning to maintain their functionality and longevity. Advanced cleaning technologies, such as automated systems or harsh disinfectants, may not always be compatible with certain materials or components of these devices, leading to potential damage. Improper cleaning methods can degrade the devices or cause malfunctions, which could result in costly repairs or replacements. Moreover, any damage to critical medical equipment raises patient safety concerns, which may deter healthcare facilities from adopting new cleaning technologies. As a result, the risk of equipment damage remains a significant barrier for many healthcare providers considering the transition to more advanced cleaning systems, limiting the growth of the medical device cleaning market.
SEGMENT ANALYSIS
BY DEVICE
The Semi-Critical segment held the largest revenue share of about 47% in the Medical Device Cleaning Market in 2023. This is due to the widespread use of semi-critical devices like endoscopes and respiratory equipment, which require frequent cleaning to prevent infection. These devices play a vital role in patient care, necessitating stringent cleaning practices. The increasing demand for infection control and safety in healthcare settings has further driven market growth in this segment.
The Critical segment is expected to grow at the fastest CAGR of approximately 11.55% from 2024 to 2032. This growth is fueled by the increasing complexity of surgeries and medical procedures that involve high-risk, critical devices. The need for advanced cleaning solutions for surgical instruments and implants is escalating. Stricter regulations and a focus on infection prevention will continue to drive the expansion of this segment.
BY TECHNIQUE
The Disinfection segment held the largest revenue share of about 53% in the Medical Device Cleaning Market in 2023. This dominance is driven by the need to ensure patient safety by preventing infections through effective disinfection of medical devices. With a wide range of semi-critical devices requiring routine disinfection, the demand for these solutions remains high. This widespread application in healthcare settings has solidified the segment’s leading position.
The Sterilization segment is expected to grow at the fastest CAGR of about 11.84% from 2024 to 2032. The increasing complexity of medical procedures drives the need for high-level sterilization of critical devices like surgical instruments. Stricter infection control regulations and a heightened focus on patient safety are boosting the demand for advanced sterilization technologies. This combination of factors positions sterilization for rapid market growth in the coming years.
BY EPA CLASSIFICATION
The Intermediate Level segment held the largest revenue share of about 52% in the Medical Device Cleaning Market in 2023. This is due to the widespread use of semi-critical devices like endoscopes, which require intermediate-level cleaning. These devices are essential in healthcare settings, where infection control is a priority. The growing adoption of such devices has driven demand for effective intermediate-level cleaning solutions.
The High-Level segment is projected to grow at the fastest CAGR of approximately 11.35% from 2024 to 2032. This growth is driven by the increasing need for high-level disinfection of critical medical devices, such as surgical instruments. As healthcare procedures become more advanced, the demand for thorough cleaning to ensure patient safety rises. Stricter regulations around infection control are further boosting the adoption of high-level cleaning methods.
REGIONAL ANALYSIS
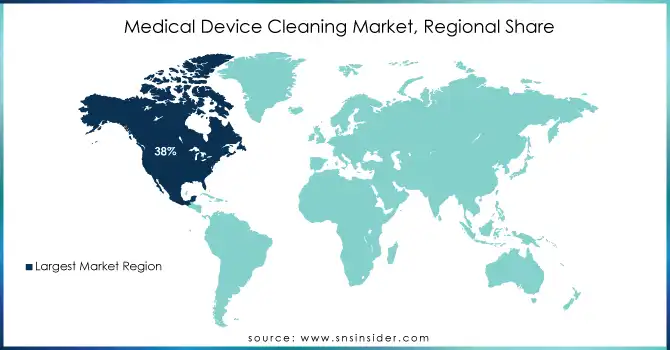
North America dominated the Medical Device Cleaning Market with the highest revenue share of approximately 38% in 2023. This is primarily due to the region’s advanced healthcare infrastructure, high adoption of medical devices, and stringent infection control regulations. The increasing focus on patient safety and the growing prevalence of chronic diseases has driven demand for effective cleaning solutions.
Asia Pacific is expected to grow at the fastest CAGR of about 12.69% from 2024 to 2032, driven by rapid healthcare expansion and rising awareness of infection control. As healthcare access improves and medical device usage increases across emerging markets, the need for reliable cleaning solutions is rising. Additionally, supportive government initiatives, growing investments in healthcare infrastructure, and rising disposable incomes are fueling market growth, positioning Asia Pacific as the fastest-growing region in the medical device cleaning sector.
Get Customized Report as per Your Business Requirement - Enquiry Now
LATEST NEWS-
-
In July 2023, Olympus launched the ETD Endoscope Washer Disinfector, a new addition to its infection prevention portfolio, designed to improve sustainability and efficiency in endoscope reprocessing.
-
In May 2023, Ecolab Inc. introduced Exelerate SCS, an enzyme-based cleaning solution for flexible endoscopes that enhances cleaning effectiveness and reduces turn-around time.
-
In April 2023, Cantel Medical Corp. expanded its endoscope cleaning and disinfection solutions by acquiring Aexis Medical, a company specializing in automated endoscope reprocessing equipment.
KEY PLAYERS
-
Steris plc (STERIS System 1, Amsco Sterilizer)
-
Getinge AB (Getinge 46 Series, Getinge 90 Series)
-
Advanced Sterilization Products (Sterrad Systems, ASP Evotech)
-
The Ruhof Corporation (Ruhof Ultrasonic Cleaner, Envirocide Disinfectant)
-
Metrex Research, LLC (Metrex CaviWipes, CaviCide)
-
Biotrol (Biotrol Healthcare Disinfectant, Biotrol Enzymatic Cleaner)
-
Oro Clean Chemie AG (Orozyt, Orosafe)
-
Cantel Medical Corporation (Cidezyme Enzymatic Cleaner, Virox 5)
-
Ecolab (Ecolab Peroxide Disinfectant, Ecolab Surgical Instrument Cleaner)
-
Belimed AG (Belimed Washer Disinfectors, Belimed Sterilizers)
-
Dr. Weigert GmbH & Co. KG (Negovent, Neodisher)
-
Zep Inc. (Zep Citrus Cleaner, Zep Heavy Duty Cleaner)
-
Hoffmann GmbH (Hoffmann Washer Disinfectors, Hoffmann Steam Sterilizers)
-
Sakura Global Holding Company Ltd. (Sakura Finetek Tissue-Tek, Sakura Tissue Processing Systems)
-
Borer Chemie AG (Borer Ultrasonic Cleaners, Borer Cleaning Agents)
-
Fortive Corporation (Fortive Chemistries, Fortive Cleaners)
-
CISA Group (CISA Sterilization Equipment, CISA Washing Systems)
-
Olympus Corporation (Olympus OER-4, Olympus Endoscope Reprocessor)
-
PAUL HARTMANN AG (HARTMANN Disinfection Wipes, HARTMANN Sterilization Packaging)
-
B. Braun Melsungen AG (B. Braun Sterilizers, B. Braun Cleaning and Disinfection)
-
Case Medical, Inc. (Case Medical Cleaner, Case Medical Sterilization Pouches)
-
Certol International, LLC (Certol Enzymatic Cleaner, Certol Enzyme Cleaner Disinfectant)
-
Tristel Solutions Ltd. (Tristel Fuse, Tristel Jet)
-
Amity International (Amity Ultrasonic Cleaner, Amity Instrument Cleaner)
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 21.74 Billion |
| Market Size by 2032 | USD 51.96 Billion |
| CAGR | CAGR of 10.22% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Device (Non-critical, Semi-critical, Critical) • By Technique (Cleaning, Disinfection, Sterilization) • By EPA Classification (High Level, Intermediate Level, Low Level) • By End User (Hospitals and Clinics, Diagnostic Centers, Dental Hospitals and Clinics, Ambulatory Surgical Centers) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Steris plc, Getinge AB, Advanced Sterilization Products, The Ruhof Corporation, Metrex Research, LLC, Biotrol, Oro Clean Chemie AG, Cantel Medical Corporation, Ecolab, Belimed AG, Dr. Weigert GmbH & Co. KG, Zep Inc., Hoffmann GmbH, Sakura Global Holding Company Ltd., Borer Chemie AG, Fortive Corporation, CISA Group, Olympus Corporation, PAUL HARTMANN AG, B. Braun Melsungen AG, Case Medical, Inc., Certol International, LLC, Tristel Solutions Ltd., Amity International. |
| Key Drivers | • Rising Awareness and Need for Infection Control Driving Medical Device Cleaning Market Growth • Technological Advancements Revolutionizing the Medical Device Cleaning Market |
| RESTRAINTS | • Risk of Equipment Damage Hindering Adoption of New Cleaning Technologies in the Medical Device Market |

