Sterile Injectables CDMO Market Size Analysis
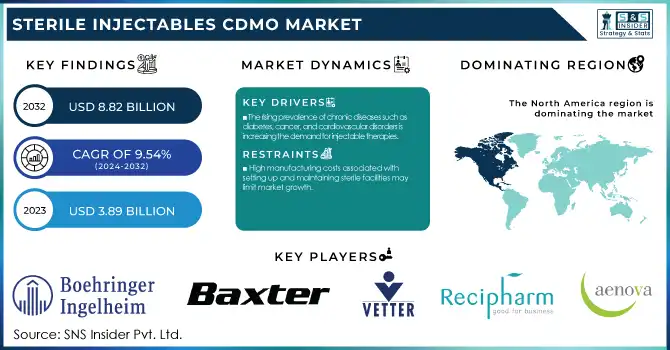
The Sterile Injectables CDMO Market Size was valued at USD 3.89 billion in 2023 and is expected to reach USD 8.82 billion by 2032, growing at a CAGR of 9.54% over the forecast period 2024-2032.
The Sterile Injectables CDMO Market Report covers significant statistical data and emerging trends based on production, regulatory, and demand drivers. The analysis explores both production volume and capacity utilization and evaluates the fill-finish capabilities of top CDMOs. It also analyses approvals, compliance performance metrics, global certificates as well as FDA and EMA certification reported in the report.
To Get more information on Sterile Injectables CDMO Market - Request Free Sample Report
It analyzes contract manufacturing across various regions to identify different outsourcing hubs. This comprehensive analysis provides a data-driven perspective on the evolving sterile injectables CDMO landscape. The Sterile Injectables CDMO Market is experiencing significant growth driven by increasing demand for complex biologics, monoclonal antibodies, and gene therapies. There was a far broader pipeline, as 55 new drugs were approved in 2023 alone by the U.S. Food and Drug Administration (FDA) for properties of several injectables.
Sterile Injectables CDMO Market Dynamics
Drivers
-
The rising prevalence of chronic diseases such as diabetes, cancer, and cardiovascular disorders is increasing the demand for injectable therapies.
The rising incidence of chronic diseases, especially diabetes and cardiovascular disorders, remains the top factor driving the Sterile Injectables Contract Development and Manufacturing Organization (CDMO) market. Recent data indicates that the number of individuals diagnosed with diabetes surged from 200 million in 1990 to 830 million by 2022, affecting approximately one in seven adults globally. Notably, India bears a substantial burden, with an estimated 212 million people living with diabetes, accounting for 26% of the global diabetic population. Cardiovascular diseases also present a growing concern. Cardiovascular diseases are another cause for concern. Ischemic heart diseases accounted for 20.5 million deaths globally in 2019, according to the latest data by the World Health Organization in 2021, for 13% of all global deaths.
In India, hypertension and diabetes together account for approximately 68% of all chronic diseases. The economic burden is notable, with 64% of patients reporting significant financial challenges due to ongoing care costs, including hospital visits and medications. The rising incidence of these chronic diseases has driven the demand for injectable therapies to rise. This trends are reinforced by heavy investments from pharmaceutical companies. For example, Eli Lilly said it was spending USD 3 billion to expand its manufacturing facility in Wisconsin to accommodate the rising demand for its injectable diabetes and obesity treatments, Mounjaro and Zepbound, which together brought in USD 4.4 billion in sales in the third quarter of 2024.
Restraint:
-
High manufacturing costs associated with setting up and maintaining sterile facilities may limit market growth.
The production of sterile injectables is inherently complex and resource-intensive, primarily due to stringent regulatory requirements and the need for advanced manufacturing facilities. Compliance with Good Manufacturing Practices (GMP) necessitates rigorous validation, continuous monitoring, thorough auditing, and meticulous documentation throughout the production process. These stringent standards are essential to ensure product safety and efficacy but significantly elevate operational costs. This financial burden can be particularly challenging for smaller manufacturers, often hindering their market entry or sustainability. Between 2020 and 2022, over 30 small sterile injectables manufacturers in Europe and Asia either ceased operations or were acquired by larger competitors, citing high compliance costs as a major barrier. Additionally, the complexity of sterile manufacturing processes contributes to drug shortages. In 2023, there was a 91% shortage of generic sterile injectables, with 58% being injectable drugs that newly went into shortage that year. These shortages are often attributed to supply chain disruptions and the intricate nature of sterile production. Consequently, the high manufacturing costs associated with sterile injectables not only limit the entry of smaller manufacturers but also contribute to supply shortages, impacting patient access to essential medications.
Opportunity:
-
Expansion into emerging markets with growing healthcare infrastructure and increasing pharmaceutical investments presents significant growth potential.
The Sterile Injectables Contract Development and Manufacturing Organization (CDMO) market is experiencing robust growth with emerging markets also expanding their healthcare infrastructures and increasing pharmaceutical investments. This strong growth trend is heavily backed by significant investments, including the recent investment of $200 million by Amgen to establish a technology center in Hyderabad, India. This facility aims to employ around 2,000 individuals by the end of the year, focusing on leveraging artificial intelligence and data science for drug development.
In China, the pharmaceutical sector is undergoing an impressive transformation, with numerous local companies introducing "super me-too" drugs, or “biobetters” aimed to treat diseases such as obesity or cancer more effectively than existing ones. Western pharmaceutical companies like GSK, Merck, and AstraZeneca have signed billion-dollar deals to gain development and distribution rights to those drugs outside of China. The reason for the surge in partnerships is that its faster and less-regulated trials provide a significant advantage over their Western counterparts. Egypt's pharmaceutical sector, one of the nation's oldest strategic industries, has expanded fivefold over the past two decades. The number of pharmaceutical factories increased from 130 in 2015 to 170 in 2023, marking a 30.8% rise. Production lines also saw a 40% increase, from 500 in 2015 to 700 in 2022. This development underlines Egypt’s position as a major contributor to international pharmaceutical supply networks.
Challenge:
-
Navigating complex and stringent regulatory requirements poses significant challenges for market participants.
The regulatory environment is becoming increasingly complex and strict, creating a challenge for CDMOs that focus on sterile injectables. For example, the U.S. Food and Drug Administration (FDA) and other regulatory entities impose strict regulations to guarantee the safety, efficacy, and quality of sterile injectable products. These standards require significant investment in advanced aseptic processing technologies, high-end cleanroom environments, and robust contamination control strategies. For instance, a recent FDA inspection of Thermo Fisher Scientific's Greenville plant identified 17 deficiencies related to sterility assurance, including inadequate sterilization of equipment and insufficient visual inspections for particulate matter in injectable drugs. These findings highlight the imperative for CDMOs to uphold strong quality management systems and remain responsive to changing regulatory landscapes. Noncompliance can lead to shutdowns, fines, and damage to reputations. In addition to this, the global nature of pharmaceutical distribution means that CDMOs must deal with a plethora of differences in regulatory standards across countries, making compliance even more challenging. Adapting to these complex and frequently shifting regulatory frameworks requires significant time and effort, rendering regulatory compliance a constant and daunting challenge inherent within the sterile injectables industry.
Sterile Injectables CDMO Market Segmentation Analysis
By Molecule Type
In 2023, the large molecule segment dominated the market with the largest revenue share of 65%. This dominance is driven by various factors, including the rising incidence of chronic diseases and the increasing demand for biologics and biosimilars. For complex diseases such as cancer, autoimmune diseases, and rare genetic disorders, large molecules (monoclonal antibodies, proteins, and peptides) are gaining momentum in treatment. The U.S. FDA's Center for Drug Evaluation and Research (CDER) has approved 55 new, agent novel drugs in the year 2023, the majority of which were biologics. It also speaks to the increasing significance of large-molecule therapeutics in the pharmaceutical industry. Additionally, this might be because producing big molecules is more complicated and is often outsourced by pharmaceutical firms to CDMOs.
According to the American Society of Gene & Cell Therapy (ASGCT), 2,082 therapies that are currently in development fall within the category of gene therapy, which typically includes large molecules. This large pipeline of innovative therapeutics is leading to demand for specialty manufacturing capabilities offered by CDMOs. Moreover, the growing emphasis on the development of personalized medicine and targeted therapy is also promoting the growth of the large-molecule segment. CDMOs partner with experience manufacturing large molecules and add significant value to pharmaceutical companies because these therapies are frequently complex to manufacture and have exacting quality control requirements.
By Product
In 2023, the pre-filled syringes segment captured the largest market revenue share of 40%. This large market share is due to multiple factors, including the growing patient inclination toward self-administration, improved safety features, lowered potential for medication errors, etc. The pre-filled syringes are generally preferred over traditional vial and syringe combinations due to benefits such as accurate dosing, a lower risk of contamination, and enhanced convenience for both healthcare professionals and patients. These advantages have resulted in heightened uptake across multiple therapeutic areas, especially in managing chronic diseases.
The FDA says that the pre-filled syringe delivery format is a growing trend in the drug industry, with a specific increase in approvals for products that utilize this type of delivery. This is especially true in the biologics sector, where numerous new drugs are being developed for subcutaneous dosing. The increasing market penetration of biosimilars also contributes to the growth of the pre-filled syringe segment. As more biologics come off patent, there is an increasing demand for cost-effective delivery systems that can compete with established brands. Pre-filled syringes offer a competitive advantage in this market by providing a user-friendly and potentially differentiated product.
By Service
The formulation development segment held the largest share of revenue at 38% in 2023. This dominance may be due to the more complicated nature of drug molecules and the rising demand for specialized knowledge in formulating injectable products that are stable and efficacious. Formulation development is the major aspect of the drug development process, especially in the case of sterile injectables, where specific attention is given to the stability, solubility, and bioavailability of the drug. With pharmaceutical companies diversifying their product pipeline to include more complex molecules, such as biologics and gene therapies, there has been a corresponding rise in demand for a range of sophisticated formulation services.
The FDA noted that in recent years, the number of complex drug products submitted for approval has increased significantly. This trend underscores the importance of formulation development services provided by CDMOs, as many pharmaceutical companies lack the in-house expertise or resources to develop these complex formulations. Further, the segment formulation development is growing due to the rise of personalized medicine and targeted therapies. Advanced Therapeutics and Drug Delivery Systems With Their Complex Structures and the Need For Specific Delivery Systems, Advanced Therapeutics Require Unique Formulation methods for enhanced therapeutic effect and shelf-life stability, which is fuelling the demand for CDMO services in this category. Furthermore, there is now a greater emphasis on enhanced patient compliance and lower healthcare expenditure, which has further generated interest in long-acting injectable formulations. Complex formulations require advanced expertise and resources to develop, increasing demand for formulation development services from contract development and manufacturing organizations (CDMOs).
By Therapeutic area
The oncology segment dominated the market, accounting for 28% of the revenue share in 2023. Such a large market share is primarily because of the growing cancer burden and the continuous evolution in cancer treatments, especially of the targeted ones and immunotherapies. As per the World Health Organization (WHO), cancer is one of the leading causes of death globally, resulting in almost 10 million deaths in 2020. The high disease burden has resulted in significant oncologic research and development and a strong pipeline of cancer therapies. According to the U.S. National Cancer Institute, more than 1,300 cancer drugs were in development as of 2023, most of which are considered injectable biologics or targeted therapies. The current oncology pipeline, comprising a high number of drugs, are expected to create a high demand for sterile injectable manufacturing services by the CDMOs.
Additionally, with a growing emphasis on personalized medicine in the treatment of cancer, treatments have become more intricate and specialized, including CAR-T cells and antibody-drug conjugates. The manufacturing process and facilities required for many advanced therapies are highly specialized, and many pharmaceutical companies have such development and manufacturing expertise outsourced to contract development and manufacturing organizations (CDMOs). The FDA has also been eager to accelerate the development and approval of cancer therapies. Novel drug approvals increased in 2023, with a good portion of these focusing on various cancer treatments and injectable products. This regulatory environment has further coupled up the growth of the oncology segment in the Sterile Injectables CDMO market.
By End use
In 2023, the biopharmaceutical companies segment captured the highest revenue share of 43% in the market. This consideration is largely due to a growing biopharmaceutical industry as well as the increased complexity of biologic drugs, which entail unique manufacturing capabilities. According to the Pharmaceutical Research and Manufacturers of America (PhRMA), biopharmaceutical companies invested an estimated $102.3 billion in R&D in 2021, with a significant portion dedicated to the development of biologics and other complex molecules. This massive investment has resulted in a large pipeline of biologic drugs, many of which need sterile injectable manufacturing.
The FDA reports that biologics account for about 40% of all prescription drug spending and 70% of drug spending growth in the United States. This trend reflects biopharmaceutical companies' increasing role in the healthcare ecosystem and their growing need for specialized manufacturing services. Moreover, the emergence of biosimilars has opened new doors for CDMOs across the biopharmaceutical landscape. The expiration of patent protections on biologics has also spurred demand for low-cost manufacturing solutions to produce biosimilars and is expected to fuel continued growth in this segment.
Sterile Injectables CDMO Market Regional Insights
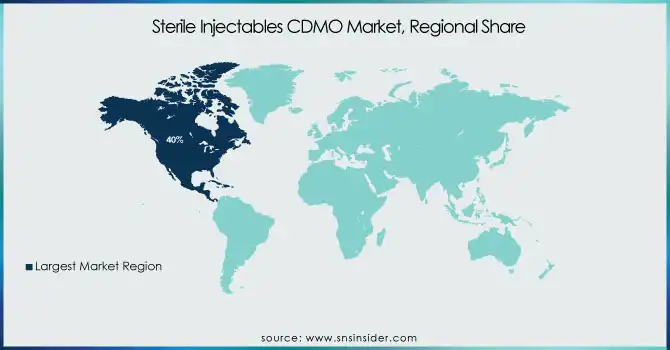
In 2023, North America held the largest market share of around 40% of the Sterile Injectables CDMO market. The region has a well-established healthcare infrastructure, large R&D investments, and several leading companies in the pharmaceutical and biotechnology sectors. The United States is the largest market for pharmaceuticals, representing roughly 45% of the total global market, according to the U.S. Department of Commerce. The changing demographics of North America with an aging population that requires oncological and urological therapies Further, with a significant market size, strong regulatory framework, and innovation ecosystem are the factors hindering fragile drug delivery, particularly in North America. In 2023, the FDA's Center for Drug Evaluation and Research (CDER) approved 55 novel drugs, with a substantial portion of these programs coming to market in injectable form. This high rate of approvals for drugs is consistent with a strong pipeline of innovative therapies in the region and is driving increased demand for sterile injectable manufacturing services.
Asia-Pacific is projected to register the highest CAGR during the forecast period. The region is experiencing this rapid growth due to factors such as the growing pharmaceutical market, increasing healthcare expenditure, and a greater focus on biosimilars and generics. One country most associated with this shift is China, which is quickly becoming an important node in the transnational pharmaceutical manufacturing landscape. According to the China National Medical Products Administration (NMPA), the country approved 59 new drugs in 2023, including several biologics and injectable products. Increasing pipeline of these innovative therapies is fuelling the demand for sterile injectable manufacturing services across the region. India, known as the "pharmacy of the world," is also contributing significantly to the growth of the Sterile Injectables CDMO market in Asia-Pacific. With a solid foundation in generic drug production and a growing interest in biosimilars and sterile injectable production, the country has become a fertile ground for emerging CDMO opportunities.
Get Customized Report as per Your Business Requirement - Enquiry Now
Sterile Injectables CDMO Market Key Players
Key Service Providers/Manufacturers
-
Baxter (Simtra BioPharma Solutions)
-
Vetter Pharma International GmbH
-
Recipharm AB
-
Fresenius Kabi Contract Manufacturing (Fresenius Kabi AG)
-
Unither Pharmaceuticals
-
FAMAR Health Care Services
-
Ajinomoto Bio-Pharma
-
PCI Pharma Services
-
IDT Biologika GmbH
-
Alcami Corporation
-
Fareva Group
-
Eurofins Scientific
-
Siegfried AG
-
Torbay Pharmaceuticals
-
Pfizer CentreOne (Pfizer Inc.)
-
ACS DOBFAR
-
Ardena Holding NV
-
Argonaut Manufacturing Services
Recent Developments in the Sterile Injectables CDMO Market
-
In November 2024, Catalent acquired a state-of-the-art biologics manufacturing facility in Princeton, NJ. It expands Catalent's large molecule sterile fill-finish services offering.
-
In October 2023, Samsung Biologics announced that its fourth manufacturing plant in Incheon, South Korea, was fully operational. The new facility markedly enhances the company's capabilities in large-scale production of sterile injectable biologics.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 3.89 Billion |
| Market Size by 2032 | USD 8.82 Billion |
| CAGR | CAGR of 9.54% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product (Pre-filled Syringes, Specialty Injectables, Vials and Ampoules, Others) • By Molecule Type (Small Molecule, Large Molecule) • By Route of Administration (Subcutaneous (SC), Intravenous (IV), Intramuscular (IM), Others) • By Service (Formulation Development, Analytical and Testing Services, Manufacturing, Packaging, Storage, Others) • By Therapeutic Area (Oncology, Central Nervous System Diseases, Hormonal Diseases, Infectious Disorders, Musculoskeletal Diseases, Cardiovascular Diseases, Others) • By End-use (Pharmaceutical Companies, Biopharmaceutical Companies, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Boehringer Ingelheim International GmbH, Baxter (Simtra BioPharma Solutions), Vetter Pharma International GmbH, Recipharm AB, Aenova Group, Fresenius Kabi Contract Manufacturing, Unither Pharmaceuticals, FAMAR Health Care Services, Ajinomoto Bio-Pharma, PCI Pharma Services, IDT Biologika GmbH, Alcami Corporation, Fareva Group, Eurofins Scientific, Siegfried AG, Torbay Pharmaceuticals, Pfizer CentreOne, ACS DOBFAR, Ardena Holding NV, Argonaut Manufacturing Services. |

