Microneedle Drug Delivery Systems Market Size & Overview:
The Microneedle Drug Delivery Systems Market Size was USD 6.1 billion in 2023, expected to reach USD 11.6 billion by 2032, growing at a CAGR of 7.4% over the forecast period 2024-2032.

To Get more information on Microneedle Drug Delivery Systems Market - Request Free Sample Report
The Global Microneedle Drug Delivery Systems Market is experiencing significant growth, owing to rising incidences of chronic diseases and rising demand for effective delivery of drugs. It mentions that 71% of all global deaths are attributed to chronic diseases according to the World Health Organization (WHO) with cardiovascular diseases, cancer, respiratory diseases, and diabetes as the leading causes of death. Therefore, the demand for advanced drug delivery systems to enhance patient outcomes while minimizing healthcare costs is on the rise. According to the U.S. Centers for Disease Control and Prevention (CDC), 6 in 10 adults in the United States have a chronic disease, and 4 in 10 adults have two or more chronic conditions. Such an umbrella of these chronic diseases has propelled government initiatives to improve healthcare provisioning and a high focus on research in advanced drug delivery systems. For instance, the National Institutes of Health (NIH) has increased its funding for research on microneedle technology, allocating over $50 million in grants for related projects in the past five years.
The European Medicines Agency (EMA) has also recognized an increasing contribution of microneedles to address therapeutic outcomes in chronic disease management across Europe. They demonstrate their focus on clinical trials using microneedle technology through their investment in several funding programs in 2023 for the advancement of these technologies. Also, the increasing prevalence of chronic diseases such as diabetes and rising demand for self-administered medications in Asia-Pacific is poised to drive government expenditure on advanced drug delivery systems. The government of China invested more than $20 million in microneedles in 2023 in their efforts to improvement of healthcare infrastructure.
Microneedle Drug Delivery Systems Market Dynamics
Drivers
-
The increasing incidence of chronic conditions like diabetes and cardiovascular diseases is driving the demand for efficient and patient-friendly drug delivery methods.
-
Innovations in microneedle design and materials, such as biodegradable and dissolvable microneedles, enhance safety and expand applications.
-
Patients increasingly prefer self-administering medications at home, reducing hospital visits and healthcare costs, thereby boosting the adoption of microneedle systems.
Rapidly increasing occurrences of chronic diseases such as diabetes, cardiovascular diseases, and cancer are some of the major factors that are contributing to the growth of the microneedle drug delivery systems market. The rising burden of chronic diseases needs better and more patient-centric drug delivery techniques. Chronic diseases typically require treatment over the long term, which historically meant injectable drugs. However, the pain and discomfort related to the conventional injections may hinder patient adherence due to long-term disease management. Microneedles offer a minimally invasive alternative, delivering medications through the skin without the need for large, painful needles. They have been especially useful for diabetes, a condition that requires frequent administration of insulin. The World Health Organization (WHO) estimates that over 537 million adults were living with diabetes as of 2021, with a projected increase to 783 million by 2045. The fast-growing diabetic population is driving the need for a non-invasive alternative to needles such as microneedles.
Additionally, microneedles are being explored for vaccines, pain management, and hormone therapies, which enhances their appeal. For instance, they have developed microneedle patches for flu vaccines that provide a needle-free pain-free option for vaccination. As their technology matures, there is increasing interest in the clinical efficacy of these methods making them an attractive candidate for chronic disease management in countries such as the US. The growing inclination towards minimally invasive and self-delivery therapeutic options is also projected to propel the microneedle drug delivery system market growth.
Restraints
-
Developing effective and safe delivery systems for a wide range of therapeutics is complex, involving considerations of molecular size, stability, and formulation requirements.
-
The substantial investment required for R&D in microneedle technologies can be a barrier, especially for smaller companies with limited resources.
The high cost involved in research and development (R&D) is one of the major factors limiting the growth of the microneedle drug delivery systems market. Innovative microneedle technologies necessitate heavy initial investments in design, materials, and clinical testing. This is a lengthy and resource-demanding process that requires generations of prototyping, lab testing, and regulatory approvals before being embarked on by industry in earnest as a reliable drug delivery system. In addition, developing safe, efficacious microneedles that are compatible with many drug formulations adds to the financial burden. Smaller companies, in particular, may struggle to secure the necessary funding to support these extensive R&D efforts. This financial barrier can slow down the pace of innovation and hinder the market entry of new products. Furthermore, ongoing testing and refinement are required to overcome challenges related to patient comfort, dosage accuracy, and long-term stability, contributing to the overall cost.
Microneedle Drug Delivery Systems Market Segmentation Insights
By Type
The hollow microneedle segment held a dominant revenue share of over 26% in 2023. Reasons behind this dominance include the versatility of the segment in terms of the range of drugs and vaccines that can be administered. Hollow microneedles provide accurate control of drug volume and delivery rate and are particularly appropriate for the delivery of insulin and other biologics. The International Diabetes Federation (IDF) states that about 537 million adults around the world will live with diabetes in 2021, and this number is predicted to climb to 643 million by the year 2030. The increasing diabetic population has massively increased the requirement for efficient insulin delivery systems, which is expected to drive the growth of hollow microneedles.
In addition, devices based on hollow microneedle technology have been approved by the U.S. Food and Drug Administration (FDA) for multiple applications. As an example, a vacant microneedle system used for intradermal injection of fluids was cleared by the FDA in 2020, the FDA granted clearance to a hollow microneedle system for intradermal injection of fluids, highlighting the regulatory acceptance of this technology. Similar research focused on hollow microneedle technology has received substantial funding through the National Institute of Biomedical Imaging and Bioengineering (NIBIB) and it is both attractive and revolutionary as a drug delivery platform. These government initiatives and the increasing prevalence of chronic diseases requiring frequent drug administration have contributed to the segment's substantial market share.
By Material
In 2023, the metal material segment led the market with a revenue share of over 29%. The notable share of this market for metal microneedles can primarily be linked to the dominant biocompatibility and mechanical properties of metal microneedles, particularly stainless steel and titanium microneedles. They are therefore composed of strong and durable metal that provides for sustained delivery of medications. NIST has developed appropriate norms for the development and quality control of metal microneedles that guarantee their safety and efficacy. This regulatory support has propelled confidence in the metal-based microneedle systems among healthcare players and patients.
Ongoing investments in research by the U.S. Department of Defense for metal microneedles for rapid vaccine delivery that is promising in military situations has also fueled this space. According to the World Health Organization (WHO), global vaccination coverage has stagnated at 86% in recent years, with no significant changes since 2010. Now, however, metal microneedles could be the answer to higher vaccination rates, as they could be made pain-free and easier to administer than conventional needles. The potential of metal microneedles has also been recognized by regulatory authorities, with the European Medicines Agency (EMA) issuing guidance for their development and use within drug delivery systems. This regulatory support, in addition to the material's established record in medical devices, has resulted in a dominant market position for the metal segment.
By Application
In 2023, the drug delivery segment accounted for the largest revenue share of 35%. There are several reasons behind such a large share of the market which includes the increasing need for effective and painless methods of drug delivery especially for chronic conditions requiring repeated doses. According to the U.S. Centers for Disease Control and Prevention (CDC), as of 2020, 6 in 10 adults in the United States had a chronic disease, and 4 in 10 adults had two or more chronic conditions. The large burden of chronic diseases has driven a need for better drug delivery systems that can improve patient adherence and clinical outcomes. Microneedle-based drug delivery has received growing interest among research agencies like the National Institutes of Health (NIH), which has funded innovative microneedle projects with applications in pain control, hormone replacement therapy, and cancer control. In 2019, the NIH provided $2.3 million for a grant that would create patches that contain microneedles for chemotherapy drug delivery. Moreover, the U.S. Food and Drug Administration (FDA) has gradually increased the attention toward microneedle technology for drug delivery and published several guidance documents to stimulate the research and development of microneedle drug delivery system (MDDS)-based products. The regulatory support has bolstered the investments of pharmaceutical companies in the microneedle drug delivery systems, thereby promoting the growth of the segment.
Microneedle Drug Delivery Systems Market Regional Analysis
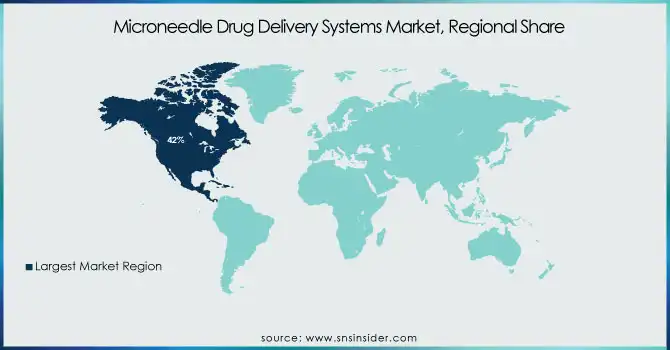
In 2023, North America led the microneedle drug delivery systems market, held around 42% of revenue share of the total market. Factors such as well-established healthcare infrastructure, high healthcare expenditure, and presence of major market players contributes to the dominance of this region. The United States, in particular, has been at the forefront of microneedle technology development and adoption. According to the Centers for Medicare & Medicaid Services (CMS), U.S. healthcare spending reached $4.1 trillion in 2020, representing 19.7% of the country's GDP. This large investment in health care has promoted the rapid implementation of new drug delivery technologies including microneedle systems. In addition, the U.S. Food and Drug Administration (FDA) has also recognized the importance of microneedle-based products, issuing guidance documents for the development of such products and approving a number of devices in recent years.
The fastest CAGR during the forecast period is projected in the Asia Pacific region. The rapid growth of this market is attributed to a number of reasons such as increasing prevalence of chronic diseases, rising healthcare expenditure, and the growing government initiatives to support healthcare access. According to the World Health Organization (WHO), the Western Pacific region, which includes many Asian countries, accounts for 39% of the global burden of chronic diseases. The high disease burden has resulted in the initiation of advanced healthcare technologies by governments in the region. For instance, the Japanese government has launched the "Healthcare 2035" initiative, which aims to promote the development and adoption of innovative medical technologies, including microneedle drug delivery systems. Similarly, the Indian Council of Medical Research (ICMR) has been supporting research on microneedle-based technology for vaccine delivery due to its potential to increase immunization coverage in the far-flung corners of the nation. The market in Asia Pacific is expected to gain high revenue growth due to these government initiatives along with the huge and growing population of this region.
Get Customized Report as per Your Business Requirement - Enquiry Now
Recent Developments
-
In January 2024, BD launched a micros needle parenteral delivery system for biologics as well it features a smart device connected to a patient smartphone to track adherence in real time and provide feedback to the provider.
-
Nanopass Technologies launched its microneedle array patch for large-molecule drug delivery in February 2024, receiving approval from the FDA for the new treatment of autoimmune diseases.
-
Vaxxas received a contract from the U.S. Department of Defense worth $50 million in March 2024, with the aim of both rapid vaccine delivery in emergencies and the development of a microneedle patch.
Key Players
-
Kindeva Drug Delivery
-
3M
-
Becton Dickinson and Company
-
AdminMed nanoBioSciences LLC
-
B. Braun
-
QuadMedicine
-
Sorrento Therapeutics (Sofusa)
-
Nanopass technologies limited
-
TheraJect Inc.
Microneedle Drug Delivery Systems Market Report Scope
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 6.1 Billion |
| Market Size by 2032 | USD 11.6 Billion |
| CAGR | CAGR of 7.4% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Type (Solid, Hollow, Dissolving, Coated, Others) • By Material (Silicon, Metal, Polymer, Others) • By Application (Dermatology, Drug Delivery, Pain Management, Cancer Therapy, Vaccine Delivery, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Kindeva Drug Delivery, 3M, Novartis International AG, Becton Dickinson and Company, AdminMed nanoBioSciences LLC, B. Braun, QuadMedicine, Sorrento Therapeutics, Nanopass technologies limited, Cardinal Health, TheraJect Inc. |
| Key Drivers | • The increasing incidence of chronic conditions like diabetes and cardiovascular diseases is driving the demand for efficient and patient-friendly drug delivery methods. • Innovations in microneedle design and materials, such as biodegradable and dissolvable microneedles, enhance safety and expand applications. |
| Restraints | • Developing effective and safe delivery systems for a wide range of therapeutics is complex, involving considerations of molecular size, stability, and formulation requirements. • The substantial investment required for R&D in microneedle technologies can be a barrier, especially for smaller companies with limited resources. |

