Toxicity Testing Outsourcing Market Size:
The Toxicity Testing Outsourcing Market Size was valued at USD 3.76 Billion in 2023 and is expected to reach USD 8.28 Billion by 2032, growing at a CAGR of 9.2% over the forecast period 2024-2032.
To Get more information on Toxicity Testing Outsourcing Market - Request Free Sample Report
The Toxicity Testing Outsourcing Market report provides key statistical insights and emerging trends, focusing on market growth projections and the increasing demand for outsourced testing services. It includes the incidence and prevalence of toxicity-related cases as it pertain to individual regions and the primary drivers behind the data. It reviews outsourcing trends including cost efficiencies and regulatory advantages, in parallel with a breakdown of toxicity testing spend across government, pharma, and CROs. Additionally, it explores the adoption of alternative testing methods, such as AI-driven models and in-vitro testing, that are replacing traditional approaches. The demand for outsourcing toxicology testing services is also influenced by factors such as rigorous regulatory requirements, the growing emphasis on drug safety, and rising expenditures on research and development. According to the U.S. Food and Drug Administration (FDA), the number of new drug applications (NDAs) and biologics license applications (BLAs) approved in 2024 reached 53, a 15% increase from the previous year. This increase in drug production resulted in a higher need for testing services for toxicity.
Toxicity Testing Outsourcing Market Dynamics
Drivers
-
Stringent regulatory mandates and safety assessment requirements compel companies to outsource toxicity testing to specialized CROs for compliance and efficiency.
Stringent regulatory mandates serve as a key driver for outsourcing toxicity testing in the pharmaceutical and cosmetic industries. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA), have intensified safety assessment requirements to protect public health. For example, in December 2024, the FDA announced proposed standard testing methods for cosmetic products containing talc to identify the presence of the carcinogen asbestos. The proposed rule would require manufacturers to analyze samples from every batch of talc-containing cosmetics using methods such as polarized light and transmission electron microscopy. Failure to comply may render products adulterated as defined under the Federal Food, Drug, and Cosmetic Act. Drug molecules (biologics and biosimilars) and related products have become more complex in the pharmaceutical industry. Sophisticated analytical testing is necessary to ensure these complex molecules are safe, pure, and effective. Since many pharmaceutical companies do not possess the equipment and expertise to conduct in vivo and even in vitro testing, they often outsource to contract research organizations (CROs) with the appropriate equipment and regulatory know-how. Additionally, this trend is prompted by the desire to comply with strict guidelines from world health authorities, such as the FDA and the European Medicines Agency (EMA). The growing dependence upon external service providers to help navigate complex regulatory environments and comply with changing safety requirements. These trends highlight that strict regulatory requirements play an important role in pushing companies to outsource toxicity testing, and toward that end, Contract Research Organizations (CROs) that specialize in safety assessment.
Restraint:
-
High costs associated with toxicology testing services can deter companies from outsourcing these essential assessments.
The high pricing of toxicology testing services acts as a key restraint in the market. This is due to the demand for advanced technologies, specialized equipment, and skilled personnel to perform thorough and precise evaluations. As an example, in vitro, toxicology testing methods used in drug discovery are becoming more prevalent because of ethical issues over animal testing, but they need complex assays and high-quality consumables. In toxicology testing, the nature of data management and analysis further drives up costs. In vitro toxicology studies have produced massive datasets that require sophisticated computational methods for their analysis. This can be expensive and a barrier to outsourcing toxicology testing in companies, as maintenance of these tools and handling of the large data needs a lot of monetary investment. Moreover, the absence of consistent protocols and methodologies throughout the industry can result in variability in testing outcomes, requiring companies to invest even more in the reliability and consistency of results. Not only this variability increase operational cost but also elongates the time taken for testing, which finally affects the complete product development pipeline efficiency.
Opportunity:
-
Expansion into alternative testing methods, such as in vitro assays and computational modeling, offers cost-effective and ethical solutions, aligning with evolving regulatory and industry trends.
The transition to the adverse outcomes pathway (AOP) and other alternative testing methods for toxicity testing, is one of the key opportunities for the global toxicity testing outsourcing market. Ethically, increasingly more scientists justify that animal testing is unnecessary, and politically, the pressure on the substitution of animals for in vitro toxicology tests grows, especially in light of recent technological advancements. Such growth is fueled by the rising use of human-based cell systems like organoids and organ-on-chip systems that have been shown to more accurately mimic human functionality compared to standard animal model systems. And, regulatory changes further reinforce this trend. As a result, the European Union introduced bans on testing cosmetics on animals, and similar policies are being contemplated worldwide. In the United States, policymakers are actively working to identify drug development approaches that lower or replace the need for animal testing. The lack of standardized data and national coordination, as seen in countries like Australia, hampers the full transition to non-animal testing methods. National-level frameworks and dedicated funding to address these issues may help facilitate the global uptake of alternative testing methods.
Challenge:
-
Ethical concerns and regulations related to animal testing pose significant challenges, prompting the need for alternative testing methodologies.
Outsourcing of toxicity testing is also challenged by ethical concerns and increasing regulations related to the use of animal testing. It is estimated that, in 2022, around 9.3 million had actual experiments performed in the EU, with a further 9.6 million bred and euthanized without experimental use total of 18.9 million animals recorded as used for scientific purposes. Notably, 49% of these procedures were classified as causing moderate to severe suffering. The efficacy of animal testing is increasingly questioned, as 95% of drugs deemed safe in animal trials fail during human clinical stages due to toxicity or inefficacy. Regulations across the world have been introduced to decrease animal testing and this discrepancy. The U.S. Environmental Protection Agency is seeking to reduce mammalian testing by 30% by 2025 and to eliminate it entirely by 2035, for example. Likewise, Spain is pursuing legislation that would give the great apes rights similar to those of humans, outlawing the use of these species in harmful experiments. As a result, companies are now forced to find alternative testing methods like organ-on-chip systems and next-generation in silico models to meet ethical compliance as well as ensure regulatory compliance due to increasingly stringent regulations. Adopting these approaches, along with new technologies, and providing additional staff training and resources is required, which may constitute economic and operational challenges for companies in the toxicity testing outsourcing industry.
Toxicity Testing Outsourcing Market Segmentation Analysis
By Method
The in vitro segment held the largest share of 56% of the revenue in the global market in 2023. This market share is mainly driven by the rising focus on reducing animal testing, coupled with the increasing acceptance of in vitro methods due to their cost-effectiveness and reliability. The U.S. Environmental Protection Agency (EPA) reported a 30% reduction in animal testing for chemical safety assessments in 2024 compared to 2020, highlighting the shift towards in vitro methods. In addition, in 2025, the U.S. Department of Health and Human Services National Toxicology Program (NTP) reported that 70% of its toxicity testing programs currently use in vitro approaches, compared with 45% in 2020.
The report states that by 2024, 65% of all toxicity data submitted for REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) compliance was sourced from in vitro studies, compared to 52% in 2022, according to the European Chemicals Agency (ECHA). Additionally, an FDA initiative to encourage the use of human-relevant in vitro models also has contributed to a 25% increase in the acceptance of in vitro data for drug safety assessment in 2024 versus 2022. In vitro approaches have also been widely adopted in pharmaceutical and chemical sectors due to the cost efficiency associated with in vitro methods, the NIH estimating that toxicity testing is 40% less expensive with these methods.
By GLP Type
In 2023, the GLP segment held the largest market share. The reason for this dominance is the stringent regulatory requirements enforced by government agencies around the world related to ensuring the quality and integrity of non-clinical laboratory studies. GLP inspections conducted in 2024 were up 15% over 2022, according to the U.S. Food and Drug Administration (FDA), signaling the increasing relevance of GLP compliance in toxicity testing. Additionally, the European Medicines Agency (EMA) announced that 95% of all toxicology data submitted for drug approvals in 2024 were from GLP-certified studies, up from 88% in 2022.
The U.S. Environmental Protection Agency (EPA) doubled down on its GLP requirements, with a 2024 report revealing that 98% of toxicity data used for regulatory decision-making was obtained from GLP-compliant studies, a 5% increase over 2022. In 2025, the National Institute of Environmental Health Sciences (NIEHS) dedicated $50 million to strengthen GLP capabilities in US centers for toxicology research, marking a 25% increase over 2023. This investment demonstrates the government’s commitment to continue setting the highest standards for testing the toxicity of chemicals. In addition, 85% of the countries reported the implementation by the OECD in 2024 of more advanced GLP monitoring programs. This factor is anticipated to contribute to the growth of this segment in the global market.
By end use
The pharmaceutical & biopharmaceutical companies segment led the market and accounted for a revenue share of 62% in 2023. The major typical market share owes to the increasing investment in drug development and the growing pipeline of pharmaceutical products. A 12% rise in the number of investigational new drug (IND) applications was reported by the U.S. Food and Drug Administration (FDA) in 2024 compared with 2023, reflecting the continuing health of the pharmaceutical research enterprise. In 2024, the pharmaceutical industry spent $102 billion on research and development, a 5.8 percent increase from the year prior, according to the National Institutes of Health (NIH). 75% of all marketing authorization applications for new drugs included comprehensive toxicology data sourced from testing services in 2024, compared with 68% in 2023, according to the European Medicines Agency (EMA). This trend underscores the increasing dependence of pharmaceutical companies on specialized toxicity testing providers. Moreover, the number of drugs in clinical development reached a historical high of 6,000 in 2024 an increase of 7% from 2023 according to the Biotechnology Innovation Organization (BIO), driving the need for toxicity testing services even higher. The U.S. Bureau of Labor Statistics estimates that jobs relating to pharmaceutical research and development will grow 17% from 2024 to 2034, highlighting the growth of the sector and its influence on the outsourcing market for toxicity testing.
Regional Insights
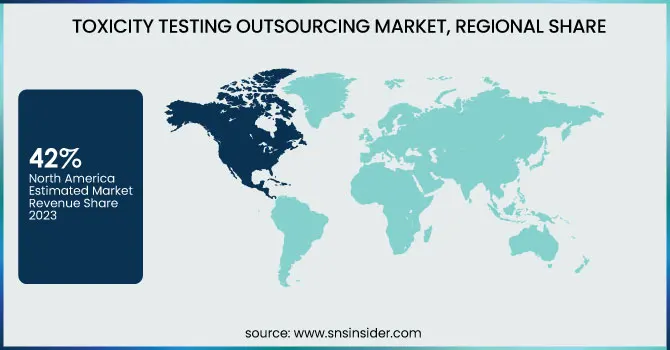
North America held the largest share in the Toxicity Testing Outsourcing Market, In 2023, North America accounted for a 42% market share, largely owing to its strong pharmaceutical industry and rigorous regulatory landscape. Demand for toxicity testing services is being driven by a 10% increase in new drug approvals in 2024 over 2023 reported by the U.S. Food and Drug Administration (FDA). In 2025, the National Institutes of Health (NIH) spent USD 43.5 billion on biomedical research, which was USD 1.4 billion or 3.5% higher than in 2024 continuing to facilitate regional market growth.
The Asia-Pacific region is expected to grow with the fastest-growing annual growth rate during the forecast period from 2024 to 2032. The rapid growth is attributable to rising investment in pharmaceutical R&D, combined with pro-government initiatives. This could explain the reported 20% new drug clinical trial applications submitted to the China National Medical Products Administration (NMPA) in 2024 compared to 2023. Meanwhile in India, the Department of Biotechnology announced an investment of USD 1.5 billion into biotech research for the period 2025-2030, a 25% increase from its previous five-year plan. Pharmaceutical exports from Japan, rising by 15% in 2024, according to Japan's Ministry of Health, Labour and Welfare, is yet another indication of activity in the industry. Owing to these factors and relatively lower operational costs and an abundance of talent pool, there has been a rapid emergence of toxicity testing outsourcing in the Asia-Pacific region.
Get Customized Report as per Your Business Requirement - Enquiry Now
Key Players in the Toxicity Testing Outsourcing Market
Key Service Providers/Manufacturers
-
Eurofins Scientific (In Vitro Toxicology Services, DiscoveryAI)
-
SGS SA (Preclinical Safety Testing, In Vitro Toxicology Testing)
-
Charles River Laboratories (Safety Assessment Services, In Vivo Toxicology Studies)
-
Thermo Fisher Scientific, Inc. (Cell-Based Assays, ADME-Tox Services)
-
Intertek Group plc (Regulatory Toxicology Services, Ecotoxicology Testing)
-
Catalent, Inc. (Preclinical Toxicology Testing, Drug Metabolism Studies)
-
ICON plc (Nonclinical Safety Assessment, Toxicokinetic Studies)
-
Medpace (Preclinical Toxicology Services, Safety Pharmacology)
-
WuXi AppTec (In Vitro Toxicity Testing, In Vivo Toxicology Services)
-
Labcorp Drug Development (Preclinical Toxicology Services, Genetic Toxicology Testing)
-
Element Materials Technology (Materials Testing Services, Certification Schemes)
-
IQVIA (Clinical Trial Execution Services, Data Management Solutions)
-
Covance Inc. (Safety Assessment Services, Toxicology Testing)
-
Envigo (Nonclinical Safety Assessment, Reproductive Toxicology)
-
Toxikon Corporation (Extractables and Leachables Testing, Biocompatibility Testing)
-
MB Research Laboratories (In Vitro Toxicology Testing, Dermal Irritation Studies)
-
Charles River Laboratories (Safety Assessment Services, In Vivo Toxicology Studies)
-
Pharmaron (Preclinical Safety Assessment, Genetic Toxicology)
-
PRA Health Sciences (Clinical Development Services, Toxicology Testing)
-
Syneos Health (Early Phase Development, Safety Assessment Services)
Recent Developments
-
In March 2023, Agilent acquired e-MSion, a company that specializes in advanced mass spectrometry technology. e-MSion has been acquired to fill the need as part of Agilent's comprehensive analytical portfolio for biotherapeutic characterization and development.
-
In March 2024, Pace Analytical boosted its laboratory network by purchasing a facility in Lebanon, New Jersey, from Curia. This acquisition will help to expand Pace's analytical testing service offerings.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 3.76 Billion |
| Market Size by 2032 | USD 8.28 Billion |
| CAGR | CAGR of 9.2% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By GLP Type (GLP, Non GLP) • By Method (In vitro, In vivo) • By End-use (Pharmaceutical And Biopharmaceutical Companies, Academic And Research Institutes, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Eurofins Scientific, SGS SA, Charles River Laboratories, Thermo Fisher Scientific, Inc., Intertek Group plc, Catalent, Inc., ICON plc, Medpace, WuXi AppTec, Labcorp Drug Development, Element Materials Technology, IQVIA, Covance Inc., Envigo, Toxikon Corporation, MB Research Laboratories, Pharmaron, PRA Health Sciences, Syneos Health. |

