Cell Therapy Human Raw Materials Market Report Scope & Overview:
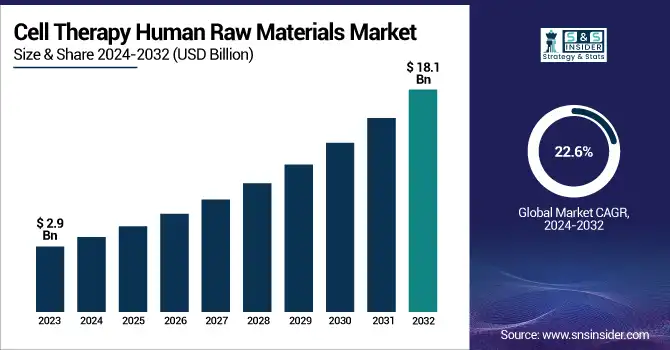
The Cell Therapy Human Raw Materials Market Size was valued at USD 2.9 billion in 2023 and is expected to reach USD 18.1 billion by 2032 and grow at a CAGR of 22.6% over the forecast period 2024-2032.
Get More Information on Cell Therapy Human Raw Materials Market - Request Sample Report
The Cell Therapy Human Raw Materials Market is the most critical segment in the biopharmaceutical industry that deals with acquiring and utilizing critical raw materials involved in the fabrication of cell therapy. The market is influenced by the growing adoption of cell-based treatments for various diseases, including cancer, autoimmune disorders, and genetic conditions. The quality and suitability of raw materials are of vital importance because they have a direct impact on the efficacy, safety, and regulatory compliance of cell therapy products.
The manufacturing of cell therapies greatly relies on the precision and purity of raw materials that include human-derived components such as blood products, tissue samples, and culture media. Because of this, the FDA has put in place stringent quality measures for the qualification of such materials to ensure safety and efficacy. In this context, general validation processes have to be carried out to avoid any form of contamination and/or any other pathogenic/contaminating agents. Assuring integrity will help to preserve the overall quality of the cell therapy product.
Key considerations in the selection of raw materials in cell therapy manufacturing involve source, quality, and consistency. PTG Lab adds there must be an assessment of the provenance of materials that are derived from human beings, which must be ethically sourced and according to rules laid down by regulators. Technology Networks adds that among the major issues manufacturers may have with sourcing are supply chain disruptions and variability in material quality, and both risk management robust strategies and supplier relationships were critical to such challenges.
According to Core Biogenesis, the system promotes the proper selection of the raw materials to achieve the highest output of cell therapy. Other aspects that will have to be considered include the stipulation of requirements for different cell therapies that involve gene therapies and stem cell treatments and the bearing of raw materials that will ensure such therapeutic outcomes. The constantly changing cell therapy landscape and the building code of regulatory requirements suggest that continuous improvement in sourcing starting raw materials and quality assurance will be required to meet future market demands.
The Cell Therapy Human Raw Materials Market is very demanding with extremely high standards for the quality and safety of materials. Innovations in raw material sourcing and management practices in the Cell Therapy Human Raw Materials Market will be driven by continuous technological development and changing regulatory landscapes.
Market Dynamics:
Drivers
-
Growing demand for cell-based treatments for chronic diseases and cancers is increasing the need for high-quality raw materials.
The huge demand for cell-based therapies for chronic diseases and cancers is one of the major driving forces in increasing the demand for high-quality raw materials in the Cell Therapy Human Raw Materials Market. Increasing medical research and clinical trials in developing cell therapies, such as CAR-T cell therapy for cancer and stem cell treatments for degenerative diseases, have led to an increased requirement for high-quality, reliable raw materials. These include, for instance, CAR-T cell therapies that require pristine leukapheresis products collected from the blood of patients, which need to be processed under very strict conditions to eventually ensure viability and potency in the engineered T cells. Similarly, stem cell therapies require culture media and growth factors free of contaminants to enable proper differentiation and functioning of stem cells. This increased focus on material quality has therefore been driven by the need to meet a raft of very demanding regulatory standards that require all components used in therapy production to be fully tested for safety, purity, and efficacy. Suppliers are, therefore, responding through increased investment in state-of-the-art purification technologies and tight quality control. Typical of this trend, companies such as Lonza and Thermo Fisher Scientific, among others, have developed a range of specialist products and services that support custom cell culture solutions through to testing regimes for the industry. The increasing complexity and scale of cell therapy production underlines critical sourcing of high-quality raw materials as part of its growing need, ensuring the success not only of therapeutic application but also advancing the entire progress of regenerative medicine.
-
Technological advancements in cell therapy manufacturing are enhancing the efficiency and reliability of raw material processing and validation.
Cell therapy manufacturing technological changes are driving a lot of efficiency and reliability in the processing and validation of the raw materials; hence, it will affect the Cell Therapy Human Raw Materials Market. Contemporary advances in the field of automated cell processing systems and advanced analytical technologies ease the complex procedures in handling and validating raw materials. Fully automated cell processing platforms that enable a range of activities, from separation to genetic modification, come with higher precision and reduced risk of human error, thus giving more consistent and reproducible results. Improved analytical tools, including next-generation sequencing and high-resolution mass spectrometry, enable the testing of raw materials with much greater depth and accuracy to ensure they will meet the extreme quality standards expected in cell therapy applications. Examples of this include advanced flow cytometry techniques and RT-PCR, which both monitor and quantify critical quality attributes in cell cultures and raw materials; thus, any deviation would be located early within the process. These technological innovations serve to further improve the general efficiency in manufacturing processes and, in turn, enhance the dependability of raw material validation as a contamination reduction means and make the final therapeutic products safe and effective. Other firms depend on digital platforms and data analytics that maximize supply chain management and predict quality issues before they could happen. This, in turn, integrated application of advanced technologies helps the industry meet the increasing demand for high-quality cell therapies by not only meeting rigorous regulatory requirements but also making further advancements in regenerative medicine.
Restrain
-
High costs and regulatory complexities associated with sourcing and validating human-derived raw materials can limit market growth.
High costs and regulatory complexities associated with sourcing and validation can hugely hamper market growth in the cell therapy human raw materials market. Procurement and verification of human-derived materials, such as components of blood or tissue samples, have huge expenses on account of the necessary stringent controls on safety and quality. These include the cost of donor material acquisition, the high cost of performing extensive testing, and the costs incurred to achieve conformity with regulatory standards. All these are heightened by the exacting regulatory environment that surrounds the use of materials sourced from humans, characterized by heavy documentation and frequent inspections aimed at assuring the application of all measures of safety. Such complex regulation can delay the approval and thus increase delays, adding to the expense. Additionally, manufacturing companies cannot continuously guarantee a supply of high-quality raw materials after overcoming all the regulatory barriers; due to this, supply chains get hindered, and the cost of production increases. Due to all these financial and regulatory burdens, scaling of operations and expansion in the market offering of a company gets hampered, which ultimately affects the overall growth and accessibility of cell-based therapies.
Opportunities
-
Innovation in raw material sourcing and the development of new, more efficient materials present significant growth opportunities in cell therapy.
The development of new raw materials and innovative approaches to sourcing raw materials have created significant avenues of growth for the Cell Therapy Human Raw Materials Market. Material science and recent improvements in biotechnology are creating new raw materials that enhance the performance and scalability of cell therapies. For instance, this may include the development of artificial or semisynthetic substitutes for human-derived materials, reducing reliance on donor sources, enabling cost reduction, and maintaining high standards of safety and efficacy. Biomaterials continue to be optimized, including engineered cell culture matrices and optimized growth factors, which could allow finer control over cell differentiation and expansion, thus yielding improvements in the efficacy of therapies. Such innovations may simplify production processes and decrease manufacturing costs to make advanced cell therapies more available. Moreover, novel materials developed with superior biocompatibility and functionality can expand indications for treatments and offer innovative therapeutic opportunities for diseases previously difficult to treat. Further technological advances in raw materials in the future will support the growth of and expand the indications of cellular treatments.
Challenges
-
Ensuring consistent quality and safety of raw materials amidst supply chain disruptions and variability remains a major challenge for manufacturers.
One of the challenges facing manufacturers in the Cell Therapy Human Raw Materials Market has to do with the variational nature of the supply chain itself: it remains a challenge to consistently assure quality and safety in supplies. Inherent therein, the diversity in biological sources of materials adds to the complexity in sourcing and managing such human-derived materials; there is also the potential for supply chain interruptions, including delays or shortages of critical supplies. For example, the irregular availability of quality donor tissues or blood products translates into variable raw materials for cell therapies that impact the reliability and safety of the final products. More important, however, supply chain disruptions caused by logistic issues, changes in regulation, or geopolitical problems may further exacerbate the challenge by delayed production and increased costs. These include strategies for quality assurance and risk management involving relationships with multiple suppliers, investments in tracking/monitoring systems at an advanced level, and flexible manufacturing practices. However, variability and unpredictability of supply continue to present major challenges to the high standards required in cell therapy development and production.
MARKET SEGMENTS
By Product
The Cell Culture Media segment dominated the Cell Therapy Human Raw Materials Market in 2023, holding an estimated market share of about 40%. This segment holds the maximum share because the cell culture media is one essential component needed to provide nutrition and environmental conditions for the growth or maintenance of cells in vitro. Quality cell culture media are essential for the realization of any successful application in cell therapy, as media support cell growth, differentiation, and functionality. For instance, special formulations of media, like those intended to be used in stem cell therapies or even in CAR-T cell therapies, need to be elaborated in very particular ways to deal with different cell types and their requirements for ideal therapeutic outcomes. The increasing demand for advanced cell therapies, combined with the requirement for customized media solutions to accommodate diverse therapeutic applications, contributes toward a big market share for this segment.
By End-Use
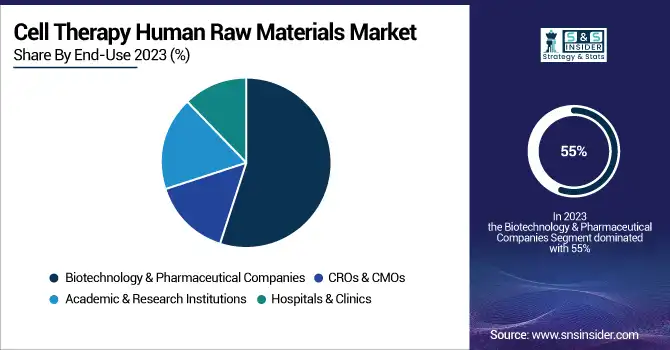
The Biotechnology & Pharmaceutical Companies dominated the Cell Therapy Human Raw Materials Market in 2023, which was estimated at 55%. Due to enormous investments and focus by these companies on the development and commercialization of cell-based therapies, the use of high volumes of high-quality raw material becomes a must in such a scenario. For example, the top biotech companies that are in the development of the latest therapies, such as gene editing and regenerative medicine, rely on very specialized raw materials that can ensure efficacy and safety. Companies active in this space generally become the driving source for modern cell culture media, growth factors, and other components required to invest extensive amounts into research, clinical trials, and manufacturing processes. All of these are empowered by large-scale production and innovation efforts in this area, creating its leading market position.
Regional Overview
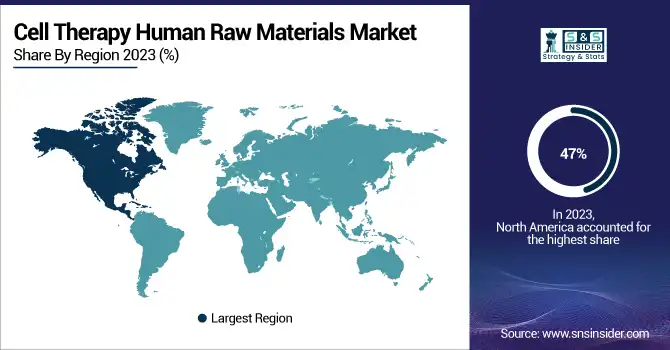
North America dominated the cell therapy human raw materials market, accounting for 47% in 2023. The dominance of this region is owed to favorable government regulatory policies, coupled with growing demand for new innovative therapeutics and increased effort from key players in the development of cell-based therapy products that target the treatment of a range of chronic disorders. In addition, the region hosts a large number of leading companies involved in manufacturing and commercialization activities related to cell-based products. This is likely to increase the demand for human raw material for cell therapy, thus driving the segment. Besides, the collaborations between major players and small biotechnology companies create more competition as companies want to grab their best positions in the fast-growing cell therapy market. Kite, a subsidiary of Gilead, inked a strategic collaboration with Arcellx, Inc. in January 2023 to develop and commercialize CART-ddBCMA for the treatment of relapsed multiple myeloma.
Need any customization research on Cell Therapy Human Raw Materials Market - Enquiry Now
Key Players
Some of the major players in the Cell Therapy Human Raw Materials Market are Thermo Fisher Scientific Inc., Lonza Group AG, Miltenyi Biotec GmbH, Stemcell Technologies Inc., Bio-Techne Corporation, BD Biosciences, GE Healthcare Life Sciences, Macopharma, NantCell Inc., CellGenix GmbH, and other key players
Recent Developments
-
January 2024: CASGEVY is Vertex Pharmaceuticals' first FDA-approved treatment for sickle cell disease using CRISPR/Cas9 cell therapy and was performed by Solvias.
-
June 2023: StemCyte partnered with a US-based cell therapy company to provide much-needed cellular raw materials needed in the development of allogeneic CAR-NK cells from umbilical cord blood.
-
July 2023: Merck KGaA invested an additional amount of USD 24.38 million to expand the production of cell culture media in the U.S., including increasing the capacity at its facility based in Lenexa.
-
March 2023: Gemini Bio opened a new cGMP bioprocess liquid manufacturing facility in West Sacramento, California, extending the capability to cater to the biotechnology and cell & gene therapy market segments.
Cell Therapy Human Raw Materials Market Report Scope:
Report Attributes Details Market Size in 2023 US$ 2.9 Billion Market Size by 2032 US$ 18.1 Billion CAGR CAGR of 22.6% From 2024 to 2032 Base Year 2023 Forecast Period 2024-2032 Historical Data 2020-2022 Report Scope & Coverage Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook Key Segments •By Product (Cell Culture Media, Cell Culture Sera, Cell Culture Supplements [Proteins, Growth Factors, Nucleotides, Others], Reagents & Buffers, Others)
•By End-Use (Biotechnology & Pharmaceutical Companies, CROs & CMOs, Academic & Research Institutions, Hospitals & Clinics)Regional Analysis/Coverage North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) Company Profiles Thermo Fisher Scientific Inc., Lonza Group AG, Miltenyi Biotec GmbH, Stemcell Technologies Inc., Bio-Techne Corporation, BD Biosciences, GE Healthcare Life Sciences, Macopharma, NantCell Inc.,
CellGenix GmbH and other key playersKey Drivers • Growing demand for cell-based treatments for chronic diseases and cancers is increasing the need for high-quality raw materials
• Technological advancements in cell therapy manufacturing are enhancing the efficiency and reliability of raw material processing and validationRESTRAINTS • High costs and regulatory complexities associated with sourcing and validating human-derived raw materials can limit market growth

