Lipid Nanoparticles Market Size & Trends:
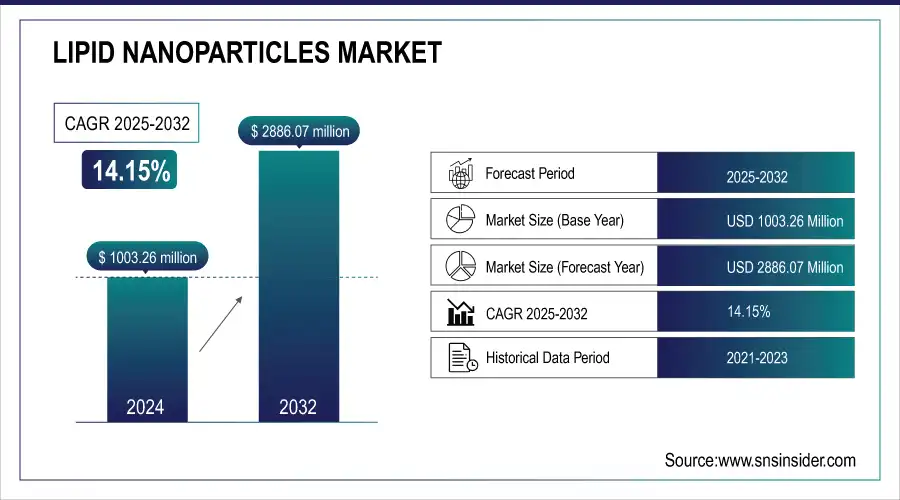
The Lipid Nanoparticles Market size was valued at USD 1003.26 Mn in 2024 and is expected to reach USD 2886.07 Mn by 2032 and grow at a CAGR of 14.15%.
The LNP market is experiencing strong growth and is primarily driven by applications in RNA-based therapies, gene therapy, and drug delivery systems. LNPs play a vital role in delivering nucleic acids such as RNA and DNA into cells, thereby assuring their stability and improving the therapeutic effects. This technology has been increasingly used in new treatments for cancer, genetic disorders, and other diseases, with a highly efficient and targeted approach to the delivery of therapy.
To get more information on Lipid Nanoparticles Market - Request Sample Report
Lipid nanoparticles are going to revolutionize cosmetic and personal care products by providing enhanced penetration into the skin and controlled release of active ingredients. These developments are going to significantly impact product performance and efficacy. Demand for cosmetic products containing lipid nanoparticles is going to increase because lipid nanoparticles are an effective treatment for anti-aging treatments and protect the skin against radiation damage.
Leveraging their antioxidant properties, cosmetics companies are increasingly using naturally derived antioxidants such as carotenoids, retinoids, and tocopherols in their skincare and therapeutic products. Formulations combining lipid nanoparticles and antioxidants have a bright future in treating skin conditions like atopic dermatitis and psoriasis by providing anti-inflammatory and irritation-reducing benefits.
In drug delivery systems, nanoparticles are expected to have better activity than the conventional free-drug delivery systems, while encouraging targeted drug accumulation by enhancing efficacy. It is likely that the circulation time, half-life, surface characteristics, the size of the particle, and degree of angiogenesis will act as crucial determinants in deciding the distribution pattern of nanoparticles in tumor tissue.
Looking forward, the market is set to continue growth as more innovations in personalized medicine occur, mRNA-based therapeutics grow in popularity, and there is a massive investment in research and development in many sectors.
Key Lipid Nanoparticles Market Trends:
• Rising adoption of lipid nanoparticles for efficient mRNA delivery in vaccines, gene therapies, and cancer immunotherapies.
• Growing investment in research and development to enhance targeted drug delivery systems with improved safety and efficacy.
• Increasing focus on personalized medicine, driving demand for precise and customizable LNP-based therapeutic platforms.
• Expanding applications of LNPs beyond infectious diseases into oncology, rare genetic disorders, and chronic conditions.
• Advancements in biotechnology enabling large-scale production and commercialization of next-generation LNP formulations.
Lipid Nanoparticles Market Growth Drivers:
-
Increased demand for mRNA-based therapies and vaccines propels Lipid nanoparticle market growth.
The growing demand for mRNA-based therapies such as gene therapies and cancer treatment is a major driver in the LNP market. Delivering mRNA into cells depends on lipid nanoparticles because they protect the mRNA against degradation. This technology, therefore, has opened avenues in the treatment of diseases that are genetic, cancers, and other complex conditions. The increased investment in the development of mRNA vaccines and cancer immunotherapy is likely to drive the LNP market further.
-
Advances in personalized medicine and an ever-increasing demand for targeted drug delivery systems are propelling the growth of the LNP market.
Personalized therapies, which are custom-made to fit the unique genetic profiles of individual patients, require highly precise and efficient drug delivery systems. LNPs offer the ideal solution, enabling the delivery of nucleic acids and other therapeutic agents directly to targeted cells with minimal side effects. The focus on gene therapies and the increased ability of biotechnology companies to produce such targeted systems is a major driver in the growth of the market. Investment in research and development in this area is expected to rise, thereby fueling the demand for LNPs.
Lipid Nanoparticles Market Restraints:
-
The high cost of producing Lipid nanoparticles.
The process involved in manufacturing LNPs makes it expensive, as it requires complex and expensive equipment and, particularly, very pure-grade lipids as raw material inputs. This is usually expensive for large-scale manufacturers and is a major challenge associated with mRNA-based therapies and gene therapies.
the complex production requirements for the LNPs, such as an exact formulation and quality controls, which make the process relatively expensive. This may make LNP-based therapies, including vaccines and gene treatments, less cheap, thereby limiting their accessibility, especially in resource-constrained environments.
In addition, as personalized medicine continues to expand and more advanced therapies are developed, the production costs of LNP-based therapeutics remain an important factor to decrease. Decreasing these costs with improved manufacturing processes and economies of scale is imperative to the goal of making such therapeutics broadly available.
Lipid Nanoparticles Market Segment Analysis:
By Application
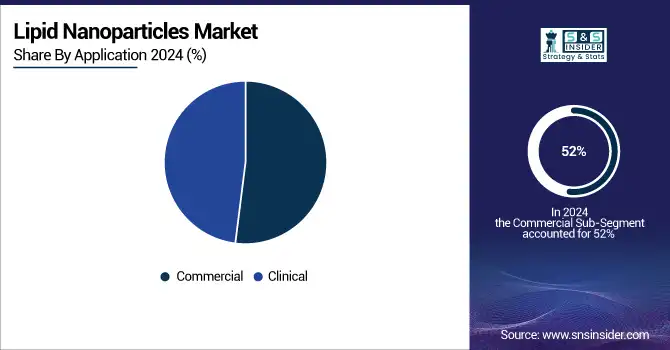
The commercial segment dominated the lipid nanoparticles market with a 52% market share driven by the increased commercialization of mRNA vaccines and RNA-based therapeutics. LNPs are also supported by demand for commercial applications through the large-scale production of vaccines, such as COVID-19 vaccines, and their entry into cancer treatment and gene therapies.
The clinical application segment is growing steadily, particularly in the development phase for gene therapies and RNA-based treatments. Although it is still smaller than the commercial segment, clinical applications are expected to grow exponentially as more therapies go from research to clinical trials, which would propel the future growth of the market.
By Product
In 2024, Ionizable lipids dominated the lipid nanoparticle market. Ionizable lipids play a vital role in the formulation of lipid nanoparticles for mRNA-based therapies and vaccines because they effectively facilitate the delivery of RNA molecules into cells. They are preferred due to their ability to improve mRNA stability and ensure efficient encapsulation, hence widely used in commercial applications. As such, they represent the core component of the landscape of mRNA vaccines and therapeutics, hence the dominant product type in the market.
PEGylated lipids are also significant and are mainly used for the improvement of stability and circulation time of lipid nanoparticles in the blood by reducing the adsorption of proteins and avoiding the quick clearance of the immune system. Even though important, they are not as dominant as ionizable lipids. Neutral lipids and phospholipids are used in specific formulations but have a more specialized role than ionizable lipids. Other formulation materials, comprising cholesterol, are used to attain optimal characteristics of lipid nanoparticles, though they have a smaller market share.
By LNP Type
The SLNs segment dominated the market with a 42% market share in 2024. SLNs have benefits such as better stability, controlled release, and the ability to deliver both lipophilic and hydrophilic drugs, making them a preferred choice for many applications. In 2024, SLNs continued to lead in mRNA vaccine production and gene therapy, with their ability to maintain the integrity of the payload, resulting in increased adoption across the pharmaceutical industry.
Meanwhile, Nanostructured Lipid Carriers is the fastest-growing segment because of their improved ability to encapsulate both lipophilic and hydrophilic drugs, thus providing greater flexibility in drug delivery systems. NLCs are increasingly being explored for personalized medicine and are gaining ground in clinical trials for new gene therapies.
Lipid Nanoparticles Market Regional Analysis:
North America Lipid Nanoparticles Market Insights
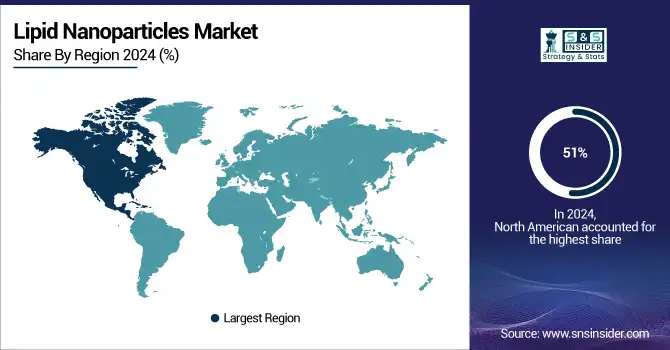
In 2024, North America dominated the lipid nanoparticles market with a market share of 51%, mainly due to its well-established pharmaceutical and biotechnology infrastructure, strong investment in healthcare, and advanced research facilities. The region has been at the forefront of LNP development, particularly in the field of RNA therapeutics and gene therapies. The successful application of LNPs in mRNA vaccines has further propelled North America's leadership in the market. North America currently holds the largest market share for LNP, mainly because of a strong presence of key pharmaceutical companies and regulatory support from bodies such as the FDA, who continue to support the development and commercialization of LNP-based products.
Get Customized Report as per Your Business Requirement - Enquiry Now
Asia Pacific Lipid Nanoparticles Market Insights
The Asia-Pacific region is one of the fastest-growing lipid nanoparticles market due to rapid development in healthcare infrastructure, rising investments in biotechnology, and a growing emphasis on advanced drug delivery technologies. LNP technologies are becoming popular among countries such as China, Japan, and India as they receive greater funding and governmental support for biotech innovation. The region's developing pharmaceutical manufacturing capabilities and increasing awareness of mRNA and RNA-based therapies are the major drivers for this rapid growth. Recent reports indicate that Asia-Pacific is expected to witness the highest compound annual growth rate (CAGR) in the LNP market with countries like China and India leading in the healthcare and biotech sectors.
In 2024, the major developments in the region, which include the expansion of pharmaceutical R&D and increasing partnerships between biopharma companies and contract manufacturers, are expected to further propel the growth of LNP applications. For example, the Australian government announced a USD 3 million grant to BioCina, a biopharmaceutical company, to support the development of LNP technologies for gene therapy applications.
Lipid Nanoparticles Market Key Players:
-
Ionis Pharmaceuticals (Vyondys 53, Waylivra)
-
CureVac (CVnCoV, CureVac's mRNA vaccines)
-
Alnylam Pharmaceuticals (Onpattro, Givlaari)
-
Moderna (mRNA-1273 (Spikevax), mRNA-1010)
-
Arcturus Therapeutics (ARCT-810, LUNAR-COV19)
-
VivoCapital (VLP-based vaccines, Lipid nanoparticle formulations for RNA delivery)
-
Novartis (Kymriah, Leqvio)
-
Pfizer (Comirnaty, BNT162b2)
-
Bayer (Vitrakvi, Kovaltry)
-
Sanofi (mRNA vaccine candidates, Dupixent)
-
Sangamo Therapeutics (SB-525, Zinc Finger Nucleases)
-
Horizon Therapeutics (Tepezza, Ravicti)
-
Genevant Sciences (GENE-012, GENE-001)
-
Enzyvant (VY-AADC, VY-GLB1)
-
Dicerna Pharmaceuticals (DCR-PHXC, DCR-HBVS)
-
Takeda Pharmaceuticals (TAK-999, Velcade)
-
Silence Therapeutics (SLN124, SLN360)
-
Revelo Genetics (RNA-based diagnostics, RNA-based therapeutics)
-
Dynavax Technologies (HEPLISAV-B, CpG 1018)
-
Translate Bio (mRNA-based therapies, TB-403)
Competitive Landscape for Lipid Nanoparticles Market:
Pfizer is a global pharmaceutical leader leveraging lipid nanoparticle (LNP) technology for mRNA-based therapeutics and vaccines. Its LNP platforms enable efficient delivery of nucleic acids, enhancing stability and targeted cellular uptake. Pfizer’s innovations, including its COVID-19 vaccine, demonstrate its expertise in LNP-driven drug delivery and advanced biopharmaceutical solutions.
-
On September 2023, Pfizer and BioNTech's FDA Approval for Updated COVID-19 Vaccine, Pfizer and BioNTech announced the FDA's approval of their supplemental Biologics License Application for the 2023-2024 formulation of COMIRNATY, targeting the Omicron XBB.1.5 variant. This vaccine received full approval for individuals aged 12 and above and emergency use authorization for children aged 6 months to 11 years.
Alnylam Pharmaceuticals is a biopharmaceutical company specializing in RNA interference (RNAi) therapeutics. The company utilizes lipid nanoparticles (LNPs) to deliver RNA-based drugs efficiently to target cells, enabling treatment of genetic and rare diseases. Its focus on innovative, targeted therapies positions it as a key player in the LNP-based drug delivery market.
-
On October 2023, Alnylam Pharmaceuticals published the APOLLO-B Phase 3 trial results for patisiran in the New England Journal of Medicine. The study highlighted its effectiveness in treating cardiomyopathy associated with transthyretin-mediated amyloidosis.
| Report Attributes | Details |
| Market Size in 2024 | USD 1003.26 Million |
| Market Size by 2032 | USD 2886.07 Million |
| CAGR | CAGR of 14.15% From 2024 to 2032 |
| Base Year | 2024 |
| Forecast Period | 2025-2032 |
| Historical Data | 2021-2023 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product (Ionizable lipids, PEGylated lipids, Neutral lipids, Phospholipids, Other Formulation Materials) • By LNP Type (Solid Lipid Nanoparticles (SLNs), Nanostructured Lipid Carriers (NLCs), Other Types) • By Molecule Type (siRNA, mRNA, Other Molecules) • By Application (Commercial, Clinical) • By End User (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, CDMOs) • By Service Type (Formulation Development Services, Manufacturing Services, Other Services) |
| Regional Analysis/Coverage | North America (US, Canada), Europe (Germany, UK, France, Italy, Spain, Russia, Poland, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Australia, ASEAN Countries, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Mexico, Colombia, Rest of Latin America). |
| Company Profiles | Ionis Pharmaceuticals, CureVac, Alnylam Pharmaceuticals, Moderna, Arcturus Therapeutics, VivoCapital, Novartis, Pfizer, Bayer, Sanofi, Sangamo Therapeutics, Horizon Therapeutics, Genevant Sciences, Enzyvant, Dicerna Pharmaceuticals, Takeda Pharmaceuticals, Silence Therapeutics, Revelo Genetics, Dynavax Technologies, Translate Bio. |

