Mesenchymal Stem Cells Market Size & Overview:
To Get More Information on Mesenchymal Stem Cells Market - Request Sample Report
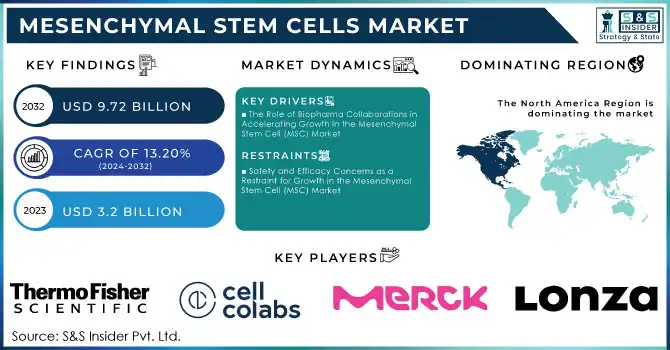
Mesenchymal Stem Cells Market was valued at USD 3.2 billion in 2023 and is expected to reach USD 9.72 billion by 2032, growing at a CAGR of 13.20% from 2024-2032.
The mesenchymal stem cell (MSC) market is growing rapidly, fueled by the rise of regenerative medicine and advancements in biotechnology. As the global population ages, the demand for regenerative therapies has increased, and MSCs have been emerging in the focus of medical studies due to anti-inflammatory and immunomodulatory benefits. Almost 3,500 MSC clinical trials are currently active as of 2023, and the attention is broadening to MSC-based treatments of chronic diseases. Regulatory support and positive outcomes from trials boost investment in MSCs by companies, making these cells an essential product in the healthcare world.
New possibilities for further development of MSC applications arise with technologies such as 3D bioprinting and the CRISPR-based gene editing technique. Furthermore, regulatory agencies are simplifying cell therapy approval procedures, which might stimulate the speedier availability of MSC-based treatments. It can be concluded that all these advances amount to a very promising future for MSCs in regenerative medicine, providing opportunities to grow and impact the healthcare industry.
MSC demand increases as more cases of degenerative diseases appear within the aging population. In 2023, 6.7 million Americans aged 65+ were living with Alzheimer's, underscoring the critical need for innovative treatments to combat age-related neurodegenerative diseases. The incredible properties of MSCs make it quite promising in solving such problems, while firms with both established pharmaceutical giants and biotech startups hence portray increased attention. Intensifying rivalry, however, is forcing firms to develop proprietary therapies using MSCs on a large scale at the same time, pushing industry progress and innovation.
Mesenchymal Stem Cells Market Dynamics
DRIVERS
-
The Role of Biopharma Collaborations in Accelerating Growth in the Mesenchymal Stem Cell (MSC) Market
Expansion in Collaboration between biotechnology and pharmaceutical companies is an important driving factor in speeding up the MSC market, improving both research and commercialization worldwide. Such partnerships effectively combine biotech innovation with pharmaceutical expertise, streamline development cycles, and even speed up regulatory approvals. The industry trends depict a growth in forming alliances in the high-demand therapeutic areas, building a stronger pipeline of MSC-based therapies. These partnerships expand patient access and market reach, reducing R&D costs while expediting market entry. As partnerships grow, they will reshape competitive dynamics, unlocking new growth opportunities in the MSC market.
-
Technological Advancements as a Catalyst for Growth in the Mesenchymal Stem Cell (MSC) Market
Technological innovations that enhance treatment efficacy, scalability, and cost-effectiveness are driving the MSC market to enormous growth. Improved cell culture, cryopreservation, and delivery mechanisms are making MSC therapies safer and far more accessible than in the past. The challenge of cell viability and storage has been minimized significantly. The cost of producing these technologies continues to decline with increased investments, which brings access to MSC treatments within reach of a larger demographic segment of patients. These advancements may not only improve the quality and reach of MSC therapies but demand dynamics are also expected to shift over time with increased competition and new opportunities for growth in regenerative medicine.
RESTRAINTS
-
Safety and Efficacy Concerns as a Restraint for Growth in the Mesenchymal Stem Cell (MSC) Market
Safety and efficacy issues are a huge restraint on the MSC market because they limit the clinical application of these therapies. Problems including immune rejection, possible tumor formation, and disparate therapeutic results altogether have led people to question the reliability and safety of MSCs. Both biological complications and technological limits, including cell quality disparities as well as the inability to control the immune response, come into play. In addition, stringent regulatory requirements and long clinical trials for addressing potential safety concerns further escalate costs and the time frame of developing MSC treatments. These safety issues thereby slow market growth as well as limit which MSC treatments will reach commercialization. This restraint can ultimately decrease market opportunities and slow the broader adoption of MSC therapies.
Mesenchymal Stem Cells Market Segment Analysis
BY TYPE
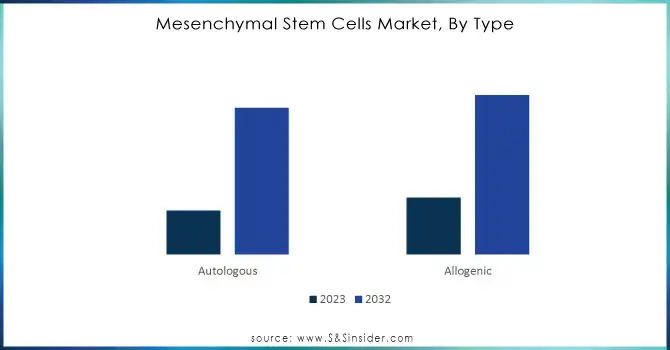
The allogenic segment dominated the mesenchymal stem cell market in 2023, taking 57% of the revenue share. This is because of established demand and technological advancement. Allogenic MSCs derived from healthy donors are off-the-shelf solutions available to treat multiple patients by reducing costs and increasing access. It also offers an advantage to address the needs of large healthcare. It is expected that continued investment into productivity and efficiency in regulatory matters shall keep allogenic as market leaders.
The autologous segment is expected to grow at a fastest CAGR of 14.43% during the forecast period from 2024 to 2032, due to growing demand for more individualized therapies. The autologous MSCs, which are derived from a patient's cells, minimize risks associated with the immune rejection process. As this segment expands with growing innovations in genetic engineering and personalized medicine, their growth will significantly shift the market dynamics of treatments, boosting investments in more differentiated therapy options.
Do You Need any Customization Research on Mesenchymal Stem Cells Market - Enquire Now
BY PRODUCTS & SERVICES
The products segment led the mesenchymal stem cell market with 80% of the revenue generated in 2023. The ready-to-use MSC therapies, for instance, in tissue regeneration and immune modulation, are in high demand. Technological improvements in cell processing and scalability have made these products more accessible and cost-efficient. This is likely to increase over time as innovation investments and regulatory approval processes make MSCs more available and efficient.
The services segment is expected to expand at the fastest CAGR of 15.65% from 2024 to 2032, as demand for personalized MSC treatments continues its upward trajectory. With the shift toward tailored therapies, the services required include cell processing and support services for clinical trials. This demand will thus open up new opportunities for specialized service providers, altering the competitive landscape and calling for more investment in patient-specific healthcare solutions.
BY WORKFLOW TYPE
The mesenchymal stem cell market was dominated by the Culture & Cryopreservation segment with a revenue share of about 46% in 2023. The high demand for reliable, scalable solutions compelled advancements in cell culture and cryopreservation techniques to improve cell viability and long-term storage capabilities, thereby making MSCs more accessible for research and clinical use. The trend is believed to continue, with increasing technological developments ensuring greater efficiency and cost-effectiveness.
The Differentiation segment is expected to grow at the fastest CAGR of 15.41% during 2024 and 2032 with innovation in regenerative medicine. As MSCs are further differentiated into functional cell types for therapeutic use, new opportunities in tissue engineering and disease modeling also emerge. Such a trend would likely attract immense investment, changing the competitive landscape and broadening the spectrum of MSC-based treatments.
BY INDICATION
The Bone Marrow segment dominated the mesenchymal stem cell (MSC) market with 44% of revenue in 2023 due to its long-standing application in regenerative medicine. Bone marrow MSCs have been extensively used to treat hematological disorders, hence strong and consistent demand. Advances in isolation and expansion technology made these cells more readily available, thereby reinstating their leading position and propelling them forward in therapeutic applications.
The Cord Blood segment would see the fastest CAGR at 15.66% from 2024 to 2032, mainly due to the potential of its applications in immune modulation and regenerative therapies. Due to the low risk of immune rejections, MSCs derived from cord blood are preferable for personal medicine. With more breakthrough research, this segment will experience high growth with increased investments and shifting market dynamics toward the consideration of cord blood as a precious source of MSCs.
Mesenchymal Stem Cells Market Regional Outlook
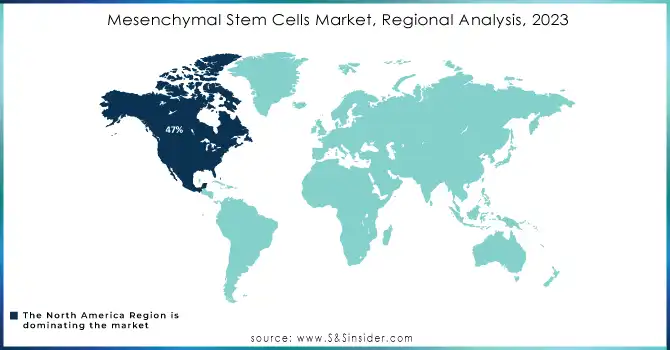
North America dominated the mesenchymal stem cell (MSC) market with the highest revenue share of about 47% in 2023, primarily driven by high demand and developed healthcare infrastructure. With the presence of key players, strong regulatory frameworks, and considerable R&D investments, the market for MSC research and therapeutic applications has been accelerated in the region. Thus, North America is expected to lead the market. It is expected to continue doing so as continuous innovation and partnerships would increase its dominance and attract more investments.
The Asia Pacific region is expected to grow at the fastest CAGR of 14.87% from 2024 to 2032, due to emerging trends in regenerative medicine and rising investment in healthcare. Increasing demand for cost-effective healthcare solutions and ever-advancing biotechnology is driving the region. This expansion is a major opportunities opportunity for the market players, shifting competition, and prompting more investments in the Asia Pacific, wherein an increasing number of developments are expected in MSC and its applications.
LATEST NEWS -
-
In 2024, Stemcell Technologies advanced Mesenchymal Stem Cell (MSC) research with their MesenCult-ACF Plus Medium, which eliminates animal components to optimize MSC culture.
-
In 2024, Thermo Fisher partnered with Mandaya Hospital Group to advance MSC production and develop CD19 CAR-T therapies using GMP-grade reagents and cell processing systems, while providing training and technical support for clinical research
KEY PLAYERS
-
Thermo Fisher Scientific, Inc. (Invitrogen Human Mesenchymal Stem Cell Kit, Gibco StemPro MSC SFM)
-
Axol Bioscience Ltd (Human Mesenchymal Stem Cells, MSC Culture Media)
-
STEMCELL Technologies (TeSR-E8 Medium, MSC-Brew XF)
-
Merck KGaA (MesoStem, CellNTech MSC Medium)
-
Lonza (Poietics Human Mesenchymal Stem Cells, XenoFree MSC Growth Medium)
-
Promocell GMBH (Human Mesenchymal Stem Cells, MSC Growth Medium)
-
Cyagen (CytoGrow™ Human Mesenchymal Stem Cells, MSC Media Kit)
-
Celprogen Corporation (Human Mesenchymal Stem Cells, CelProgen MSC Media)
-
Cellcolabs (Human Bone Marrow-Derived Mesenchymal Stem Cells, Mesenchymal Stem Cell Growth Medium)
-
Stemedica Cell Technologies, Inc. (Stemedica Human MSCs, Stemedica MSC Media)
-
Cell Applications, Inc. (Human Mesenchymal Stem Cells, MSC Growth Medium)
-
Bio-Techne Corporation (R&D Systems Human Mesenchymal Stem Cells, StemXVivo MSC Expansion Medium)
-
Biosafe S.A. (BIOCOTE MSC Cryopreservation Solution, MSC Expansion Medium)
-
Medipost Co., Ltd. (StemChord Human MSCs, Medipost MSC Expansion Medium)
-
Evoke Pharma, Inc. (Human Bone Marrow MSCs, MSC Expansion and Culture Kits)
-
Wuxi AppTec (Human Mesenchymal Stem Cells, Stem Cell Expansion Media)
-
Fujifilm Irvine Scientific (Primordial Human MSC Culture Medium, XenoFree MSC Medium)
-
Chong Kun Dang Pharmaceutical Corp. (KST-MSCs, KST-MSC Culture Kit)
-
Kirkegaard & Perry Laboratories (KPL) (MSC Growth Medium, Human Mesenchymal Stem Cell Culture System)
-
GE Healthcare Life Sciences (Xeno-free MSC Expansion Media, Human Mesenchymal Stem Cells)
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 3.2 Billion |
| Market Size by 2032 | USD 9.72 Billion |
| CAGR | CAGR of 13.20% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Products & Services (Products, Services) • By Workflow Type (Cell Sourcing & Isolation, Culture & Cryopreservation, Differentiation, Characterization) • By Type (Autologous, Allogenic) • By Source of Isolation (Bone Marrow, Cord Blood, Peripheral Blood, Fallopian Tube, Fetal Liver, Lung, Adipose Tissues) • By Indication (Bone And Cartilage Repair, Cardiovascular Diseases, Inflammatory And Immunological Diseases, Liver Diseases, Cancer, GvHD, Others) • By Application (Disease Modeling, Drug Development & Discovery, Stem Cell Banking, Tissue Engineering, Toxicology Studies, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Thermo Fisher Scientific, Inc., Axol Bioscience Ltd, STEMCELL Technologies, Merck KGaA, Lonza, Promocell GMBH, Cyagen, Celprogen Corporation, Cellcolabs, Stemedica Cell Technologies, Inc., Cell Applications, Inc., Bio-Techne Corporation, Biosafe S.A., Medipost Co., Ltd., Evoke Pharma, Inc., Wuxi AppTec, Fujifilm Irvine Scientific, Chong Kun Dang Pharmaceutical Corp., Kirkegaard & Perry Laboratories (KPL), GE Healthcare Life Sciences |
| Key Drivers | • The Role of Biopharma Collaborations in Accelerating Growth in the Mesenchymal Stem Cell (MSC) Market • Technological Advancements as a Catalyst for Growth in the Mesenchymal Stem Cell (MSC) Market |
| RESTRAINTS | • Safety and Efficacy Concerns as a Restraint for Growth in the Mesenchymal Stem Cell (MSC) Market |

