Preeclampsia Diagnostics Market Size & Trends:
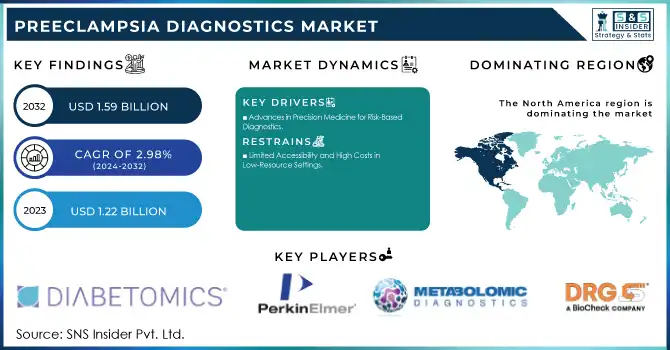
The Preeclampsia Diagnostics Market size was valued at USD 1.22 Billion in 2023 and is expected to reach USD 1.59 Billion By 2032 with a growing CAGR of 2.98% over the forecast period of 2024-2032.
To Get More Information on Preeclampsia Diagnostics Market - Request Sample Report
The Preeclampsia Diagnostics Market is experiencing robust growth, driven by advancements in diagnostic technologies and increasing awareness of maternal health risks. Preeclampsia, a hypertensive disorder affecting 2%–8% of pregnancies globally, remains a leading cause of maternal and neonatal mortality. The condition’s rising prevalence, attributed to factors such as obesity, advanced maternal age, and pre-existing health conditions like hypertension and diabetes, underscores the urgent need for early and accurate diagnostic solutions.
Recent advancements in diagnostic technologies have significantly enhanced early detection capabilities. For instance, biomarkers such as placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) are used in diagnostic assays, demonstrating high sensitivity and specificity in predicting preeclampsia. Non-invasive testing methods, including blood-based and urine-based assays, have gained traction due to their accuracy and convenience. Moreover, a study published in 2023 highlighted the use of artificial intelligence (AI)-driven predictive models, which achieved over 90% accuracy in early detection using maternal health data and biomarkers.
Public health initiatives have further contributed to market growth. For example, the establishment of World Preeclampsia Day has brought global attention to the condition, emphasizing the importance of early screening and intervention. Campaigns led by organizations like the Preeclampsia Foundation aim to educate healthcare providers and expectant mothers on recognizing symptoms and risks, fostering increased adoption of diagnostic tools.
However, challenges persist. Despite an estimated 20+ candidate diagnostics under investigation, the number of approved diagnostic products remains low. Accessibility in low-resource settings also hinders widespread adoption, where the burden of preeclampsia is often highest. Efforts to address these gaps include the development of cost-effective, point-of-care diagnostic solutions, which are expected to improve accessibility and maternal health outcomes.
Market Dynamics
Drivers
-
Advances in Precision Medicine for Risk-Based Diagnostics
The growing emphasis on personalized medicine has transformed the landscape of preeclampsia diagnostics. By leveraging genomics and proteomics, healthcare providers can now identify high-risk pregnancies with greater accuracy. These advancements facilitate the early detection and management of preeclampsia, significantly reducing complications for both mothers and infants. Tailored diagnostic solutions ensure that care is optimized based on individual risk profiles, supporting proactive healthcare measures. This personalized approach aligns with the global shift toward precision medicine, making it a pivotal driver in the market's expansion.
-
Technological Innovations in Diagnostic Solutions
Cutting-edge technologies, such as microfluidic systems and molecular diagnostics, are revolutionizing preeclampsia testing. These innovative methods deliver rapid and accurate results, meeting the critical need for timely diagnosis in point-of-care settings. The portability and cost-effectiveness of these tools make them particularly valuable in remote or resource-limited areas. As healthcare systems adopt these advanced diagnostics, they ensure better clinical outcomes by enabling swift interventions. These technological advancements underscore the industry's commitment to enhancing diagnostic efficacy.
-
AI and Investment in Maternal Health Infrastructure
Artificial intelligence and machine learning are increasingly integrated into preeclampsia diagnostics, enabling predictive analytics and early warnings for clinicians. These AI-driven platforms analyze complex datasets to identify potential risks with remarkable precision. Concurrently, investments in maternal healthcare infrastructure, supported by government and private sector initiatives, improve access to diagnostic tools globally. Collaborative programs also promote the development of low-cost, point-of-care solutions, ensuring that advanced diagnostics reach underserved populations. Together, these efforts are reshaping maternal healthcare and driving market growth.
Restraints
-
Limited Accessibility and High Costs in Low-Resource Settings
A significant restraint in the preeclampsia diagnostics market is the limited accessibility and affordability of advanced diagnostic solutions in low-resource regions. While innovative technologies like molecular diagnostics and AI-driven platforms are transforming the market, their high costs and infrastructure requirements pose challenges for widespread adoption, particularly in developing countries. Many healthcare facilities in these areas lack the necessary equipment and trained personnel to implement advanced diagnostics effectively. Additionally, the high out-of-pocket expenses for patients further limit their accessibility. Addressing this disparity requires focused efforts to develop cost-effective, point-of-care solutions and expand healthcare infrastructure in underserved regions to ensure equitable access to life-saving diagnostic tools.
Preeclampsia Diagnostics Market Segmentation Analysis
By Test Type
In 2023, blood tests emerged as the dominant segment, contributing over 60% of the market share. This dominance is attributed to their precision in detecting biomarkers like placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1), which are crucial for early and accurate diagnosis of preeclampsia. Blood tests are widely utilized in healthcare facilities due to their high reliability and the availability of standardized testing protocols. Their ability to detect the condition in its early stages ensures timely medical intervention, further cementing their leading position in the market.
Urine analysis is the fastest-growing segment, gaining popularity for its non-invasive nature and ease of administration. With innovations in diagnostic techniques such as proteinuria dipstick tests, urine analysis offers a practical solution for early detection, particularly in resource-limited settings. Its accessibility and patient-friendly approach drive its rapid adoption across healthcare facilities worldwide.
By Product
The consumables segment, including reagents, test strips, and assay kits, accounted for approximately 55% of the market share in 2023. These products are essential for conducting diagnostic tests, resulting in recurring demand from laboratories and healthcare providers. Their affordability and compatibility with various diagnostic platforms make them indispensable for blood and urine testing, reinforcing their market dominance.
Instruments, such as automated analyzers and portable diagnostic devices, represent the fastest-growing product segment. The demand for advanced instruments is rising due to their ability to deliver rapid and precise results. Their increasing adoption in point-of-care and remote healthcare settings reflects the growing need for efficient and reliable diagnostic solutions. These innovations are reshaping the market by expanding access to high-quality diagnostics.
Regional Outlook
North America led the Preeclampsia Diagnostics Market in 2023, driven by advanced healthcare systems, high maternal health awareness, and access to state-of-the-art diagnostic technologies. In the U.S., significant investments in maternal healthcare and a focus on personalized medicine have led to the widespread adoption of blood tests and innovative diagnostic instruments. Government support, including research funding and initiatives to improve maternal health, further bolsters market growth in this region.
Europe closely followed, with countries like Germany, the UK, and France at the forefront of preeclampsia diagnostics. The region’s commitment to enhancing maternal healthcare and integrating innovative diagnostic technologies into everyday clinical practice drives market expansion. A robust regulatory environment in Europe also facilitates the swift adoption of advanced solutions, including AI-powered diagnostic tools and molecular testing, which continue to gain traction in healthcare facilities across the region.
The Asia-Pacific region is rapidly emerging as a high-growth preeclampsia diagnostics market. With rising awareness of preeclampsia and increasing healthcare access, countries like India and China are seeing a surge in demand for diagnostic solutions. The growing prevalence of high-risk pregnancies, with the adoption of mobile and point-of-care diagnostic technologies, is a key factor driving market growth. Additionally, the region’s improving healthcare infrastructure is making advanced diagnostic solutions more accessible, contributing to the ongoing expansion of the market.
Do You Need any Customization Research on Preeclampsia Diagnostics Market - Enquire Now
Key Players in the Preeclampsia Diagnostics Market and Their Products
-
-
Elecsys sFlt-1 Immunoassay
-
-
PerkinElmer Inc.
-
DELFIA Xpress Preeclampsia Test
-
-
-
DRG Preeclampsia Risk Test
-
-
-
B·R·A·H·M·S KRYPTOR Preeclampsia Risk Test
-
-
Diabetomics, Inc.
-
Preeclampsia Risk Panel
-
-
Metabolomic Diagnostics Ltd.
-
Metabolomic Diagnostic Test for Preeclampsia
-
-
Sera Prognostics
-
PreTRM Test
-
-
Siemens Healthineers AG
-
Atellica IM sFlt-1 and PlGF Immunoassays
-
-
Bayer AG
-
Adiana Preeclampsia Test
-
-
Bio Rad Laboratories Inc.
-
BioPlex 2200 System
-
-
BioCheck Inc.
-
sFlt-1 and PlGF ELISA Assay Kit
-
-
bioMerieux SA
-
VIDAS Preeclampsia
-
-
Diabetomics Inc.
-
Preeclampsia Risk Panel
-
-
Sysmex Corporation
-
Sysmex Preeclampsia Test
-
-
Fujirebio Diagnostics
-
Fujirebio Pre-eclampsia Tests
-
-
Siemens Healthineers AG
-
Atellica IM sFlt-1 and PlGF Immunoassays
-
-
ACON Laboratories Inc.
-
ACON Preeclampsia Test
-
Recent Developments
In Dec 2024, Gravidas Diagnostics was awarded USD 3 million by ARPA-H to develop a home-based fingerstick test for preeclampsia. The test aims to detect sFlt‐1 levels in the bloodstream through a colorimetric assay.
In Sept 2024, Trinity Biotech plc, a biotechnology company specializing in human diagnostics and diabetes management, acquired Metabolomics Diagnostics, an Irish company focused on biomarker-based diagnostics for complex diseases. The acquisition, valued at approximately USD 1.3 million, involved over 270,000 Trinity Biotech ADS, along with cash and the assumption of liabilities.
In May 2023, CSEM’s Tools for Life Sciences team partnered with the innovative Swiss start-up MOMM Diagnostics to develop a revolutionary point-of-care solution for preeclampsia diagnostics. This advanced multiplexed sensing device was designed to simultaneously detect two key biomarkers associated with the disease.
| Report Attributes | Details |
| Market Size in 2023 | USD 1.22 Billion |
| Market Size by 2032 | USD 1.59 Billion |
| CAGR | CAGR of 2.98% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Test Type (Blood Tests, Urine Analysis) • By Product (Instruments, Consumables) • By End-user (Hospitals, Specialty Clinics, Diagnostic Centers, Others |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | F. Hoffmann-La Roche Ltd, PerkinElmer Inc., DRG Instruments GmbH, Thermo Fisher Scientific Inc., Diabetomics, Inc., Metabolomic Diagnostics Ltd., Sera Prognostics, Siemens Healthineers AG, Bayer AG, Bio Rad Laboratories Inc., BioCheck Inc., bioMerieux SA, Sysmex Corporation, Fujirebio Diagnostics, and ACON Laboratories Inc. |
| Key Drivers | • Advances in Precision Medicine for Risk-Based Diagnostics • Technological Innovations in Diagnostic Solutions • AI and Investment in Maternal Health Infrastructure |
| Restraints | • Limited Accessibility and High Costs in Low-Resource Settings |

